Analysis-ready data for informed clinical decision-making
Our solutions support clinical trial design optimization by providing structured data from published clinical literature to power intelligent data-driven insights. Our data solutions are focused on extracting and curating clinical trial outcome data, biology and pharmacology data, and pharmacokinetic data from various clinical literature.
Our services
Clinical trials outcome data (CTOD)
- For model-based meta analysis (MBMA), PBPK, disease modeling, and QSP needs
- Custom curation for various clinical trials and drug development analyses
- Data for systematic literature review (SLR) and for clinical trial simulation platforms
Intelligent quantitative systems pharmacology (iQSP)
- Comprehensive clinical, biology, and pharmacology data
- Custom curation for predicting exposure-response (efficacy/safety), target identification and validation, and biomarker identification
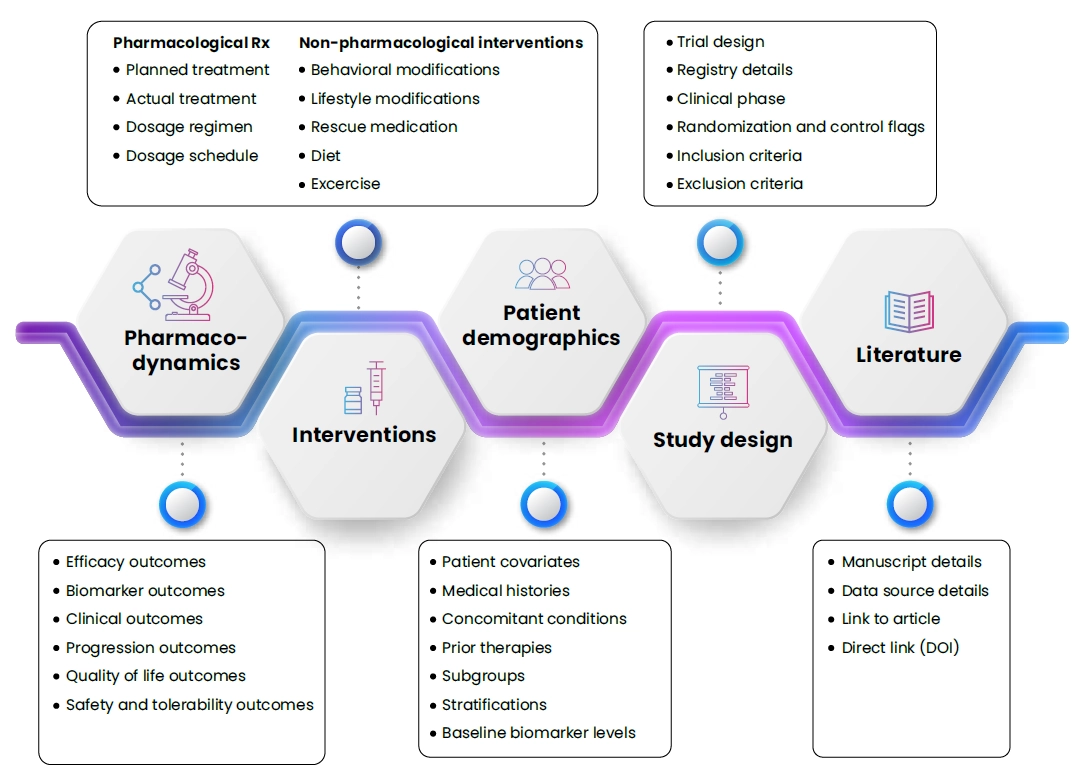
Data curation offerings for iQSP
Clinical
- Study design
- Patient characteristics and subgroups
Biology/pre-clinical/translational
- Dynamic of cells, biomolecules, and omics
- Biological processes
- Human, preclinical, and in vitro (on demand)
Pharmacology
- Rx of interest to be predicted and SOC for validation, dosage regimen
- PK (systemic and target exposure
- PD and clinical outcomes (measures of efficacy and safety)
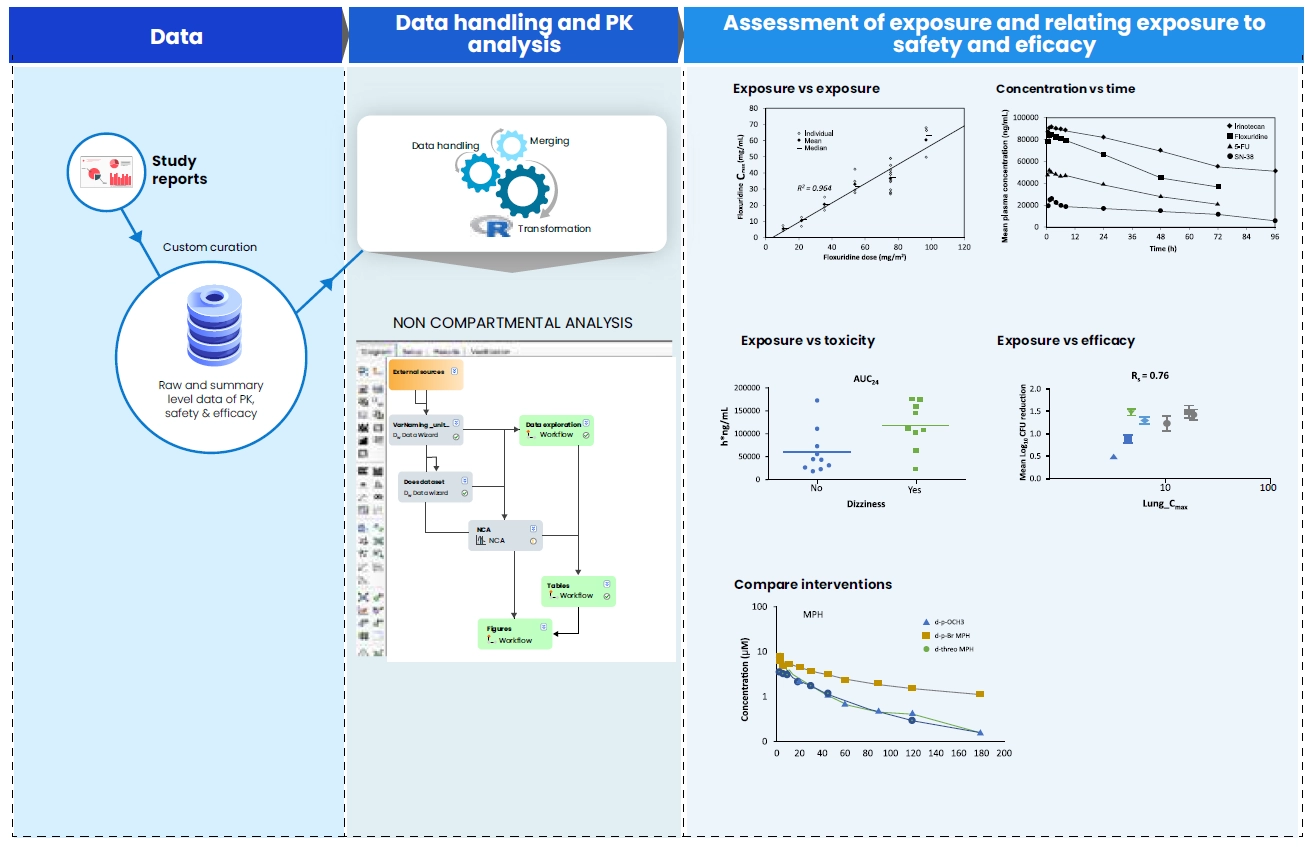
Preclinical tox report digitization
- Pharmacokinetics (PK) data
- Toxicokinetic data for safety studies
Digitization, restructuring and exploratory plots
Case study
A data-driven competitive landscape analysis to facilitate go/no-go decisions in clinical development
Our client sought to determine the competitive position of a novel antibody against rheumatoid arthritis (RA). We were engaged in supporting data needs for quantitative assessment of the longitudinal time course of clinical efficacy.
We coupled a robust scientific curation methodology with a systematic literature review (SLR) to synthesize data on existing therapies. Then we created a dataset as per client’s requirement to capture clinical outcomes summary data, patient population details, interventions, comparators, and study size.
With the digitized content, we produced a detailed landscape that allowed the client to accurately assess the validity of further development. The results showed that the novel antibody had an inferior efficacy profile in RA to existing treatments, so the client was able to save significant time and money by ceasing development at that stage.
Why Excelra
99.9
150
15
120
Ready to get more from data?
Tell us about your objectives. We’ll help get you there.
"*" indicates required fields