Table of content
- Introduction
- Basics of clinical data management
- Importance of CDM in clinical trials
- Clinical study data management: Ensuring accuracy, integrity, and compliance
- Clinical data management workflow
- Clinical data management systems
- Selecting the right CDMS
- Issues in clinical data management
- Conclusion
- Clinical Data Management & Analytics by Excelra
Introduction
Clinical data management (CDM) is the most important phase in any type of clinical research. This document includes the significance of CDM, its roles and responsibilities, generation of high-quality data. It enhances the effectiveness of the entire trial process which ensures the regulatory compliance in addition. To get a better understanding of how clinical data management affects the effectiveness and safety of novel medicines, researchers and stakeholders can investigate the intrimanagement of data in clinical trial applications of CDM.
Basics of clinical data management
The most important step in clinical research is Clinical Data Management (CDM), which is aimed at ensuring the data that is stored in clinical trials is clean, consistent, and statistically sound. It is sometimes referred to be the backbone of clinical research since it provides a constructed approach to handle ample amounts of information resulted during trials. The process requires the collection, cleaning, and management of data produced during clinical trials in conformity with regulatory guidelines. This structured approach ensures that data should be amended into meaningful understandings that can support to clinical findings.
The conversion of raw data into high-quality information that is prepared for the evaluation and regulatory body compliance can be easily done by CDM. This significant change makes it possible for clinical researchers to make sure that the accurate inferences are derived from the data which can subsequently be used to validate claims regarding the efficiency and safety of new medications. By adhering to meticulous data management guidelines, CDM helps alleviate the risks coupled with data handling, ensuring that the concluding conclusions of a clinical trial are supported by scientific evidence.
Importance of CDM in clinical trials
Because the efficacy and safety evaluations of investigational products are directly impacted by data from clinical trial, the integrity of data is very crucial for the clinical trial data. Researchers cannot draw reliable conclusions without accurate data, which could jeopardize patient safety. By ensuring consistent and accurate data collection, CDM minimizes errors and biases. To make it possible to compare the results across different studies, enhancing the reliability of research findings, consistency is very important step for the data collection.
By adhering to robust data management protocols, it enables researchers to make informed decisions that results to successful clinical outcomes. Every step of data handling, from collection to analysis, involves stringent checks and balances them. Additionally, by cutting down the time needed to validate and verify data, effective CDM practices contribute to the speed and efficiency of the drug development process. It ultimately speeds up the development of new medications, therapies, thus benefiting patients who are waiting for innovative and cutting-edge treatments.
Clinical study data management: ensuring accuracy, integrity, and compliance
To conduct the clinical trials, Clinical study data management (CSDM) portrays a key role, where the precision, integrity, and compliance of data collected, directly persuade the validity of study results. Efficient data management for clinical trials is becoming progressively more essential for achieving consistent outcomes and make sure the safety and effectiveness of the new treatments. As clinical research develops with novel technologies and regulatory demands, effective data management for clinical trials is gradually more vibrant for achieving trustworthy results and ensuring the safety and efficacy of new treatments. The modules, challenges, and best procedures in data management in clinical trials and its related projects are essentially explored in this section.
Clinical data management workflow
Clinical data management workflows are proposed to confirm the unified data collection and its processing during the clinical trial lifecycle. It includes various significant phases, each pointed at preserving the data quality and its integrity. Identifying this workflow is vital for everyone working in clinical research, since it delivers an organized methodology for overseeing trial data.
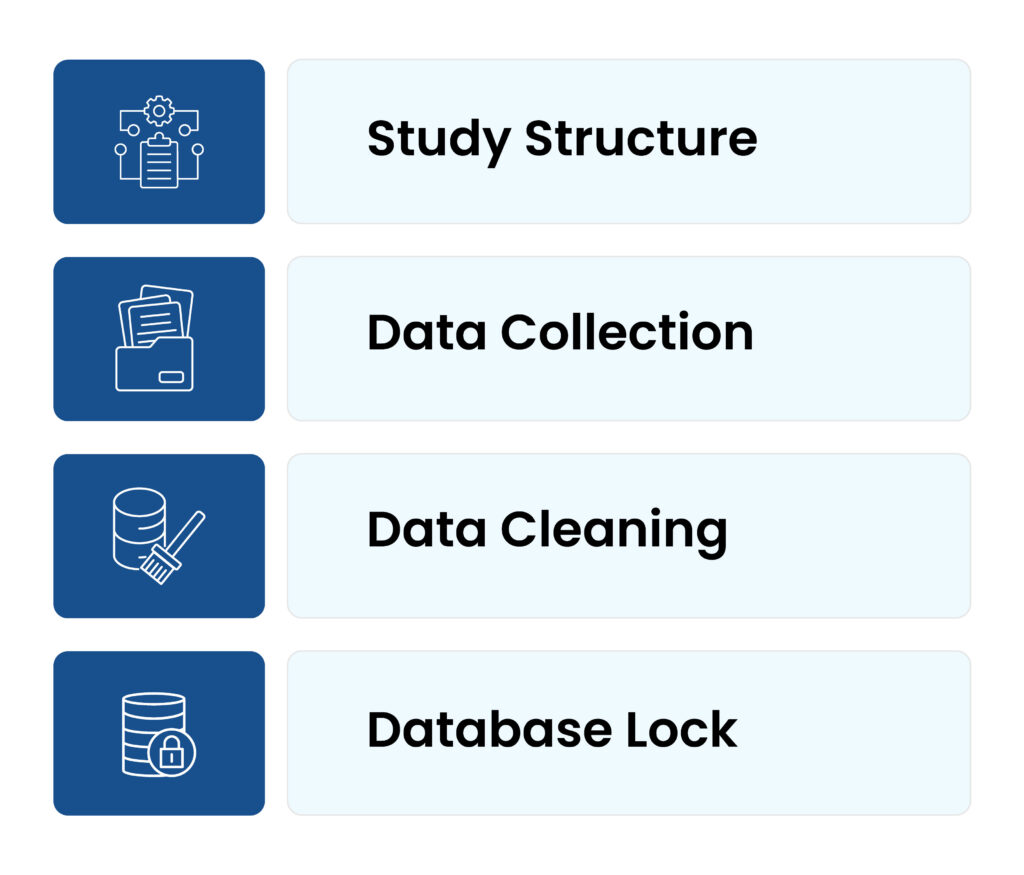
Figure 1: CDM Workflow
1. Study structure:
The study protocol and CRFs are designed in the initial phase of CDM workflow. It ensures that all necessary data points are classified and documented, setting the grounds for the intact data management procedure. To make sure that all necessary data points are documented, the Data Management Team works together with the clinical teams. This alliance is crucial, as it make sure that the data accumulated will be adequate and ample for the goals of the study.
2. Data collection:
Data is collected from numerous resources during the trial. The data includes electronic health records, research laboratory results, and patient-reported results. This stage involves comprehensive attention to detail, because any slipups in data collection could have a significant consequence for the outcome of the trials. Clinical Data Management systems allow the efficient collection and documentation of the data. Large volumes of data can be handled by these systems, ensuring that it is collected safely and is easily accessible for analysis.
3. Data cleaning:
Data cleaning is a crucial stage that involves the finding and managing the data inconsistencies. To retain the integrity of the trial results, it ensures that the data is precise and comprehensive prior to analysis. Researchers can be confident in the authenticity of their results, by focusing on errors or irregularities at this stage.
4. Database lock:
The database is secured, after the data has been thoroughly cleaned and verified, preventing any further modifications. This stage is essential for retaining the reliability of the data analysis. It indicates the point on which the data is completed and prepared for statistical analysis, assuring that the findings are centred on a stable dataset. This step is crucial for maintaining the integrity of the data analysis. By securing the database, researchers may be assured that the data they utilize for their analyses is both precise and completed.
Clinical data management systems
Software applications that are used to manage the data collection during clinical trials are known as Clinical Data Management Systems (CDMS). They streamline the whole data management process for data entry, validation, and reporting. These systems are important for ensuring data integrity because it aids effective data capture, validation, and reporting. By systematizing aspects of data management, CDMS decreases the risk of human mistake and upgrade the overall efficacy of clinical trials.
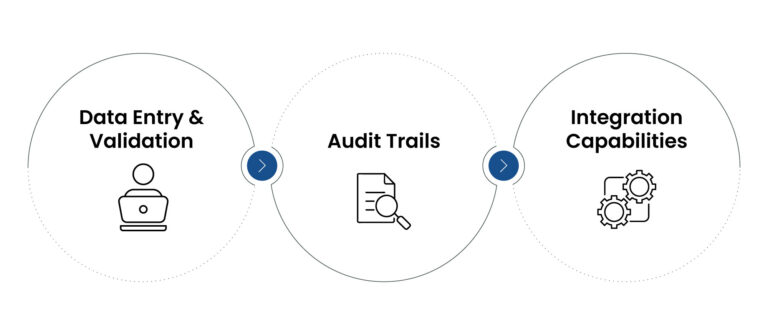
Figure 2: CDM System
Features of CDMS
1. Data entry and validation:
By utilizing the automated checks in data management, it facilitates accurate entry of the data and its validation. These checks aid to confirm that data is recorded appropriately, lowering the odds of errors that might undermine the trial results.
2. Audit trails:
It maintains a log of all the data alterations that are used for compliance and transparency. It is the most essential feature for complying regulatory constraints, because it provides an illustrated chronicle of data alterations.
3. Integration capabilities:
By allowing data transfer between numerous platforms, CDMS boosts the competency of the data management procedures. It also enables smooth data flow through integration with other systems.
Selecting the right CDMS
For a clinical trial to be successful, choosing a right CDMS is most important. While an incorrect option can result into major challenges, choosing the right system can significantly improve the efficacy and precision of data management methods. The main factors to be considered includes, ease of access, the ability to scale, and adherence to regulatory guidelines. The successful outcome of the clinical trials can be achieved, if researchers can choose a system that meets the specific needs and their requirements by evaluating the given factors.
Issues in clinical data management
Despite its crucial role, CDM encounters various number of issues that may impact the clinical trial data quality. Such challenges emerge because of the intricate and dynamical nature of the clinical research, expecting robust approaches to deliver them successfully. Knowing these issues is necessary for creating effective resolutions and confirming the successful outcome of clinical trials.
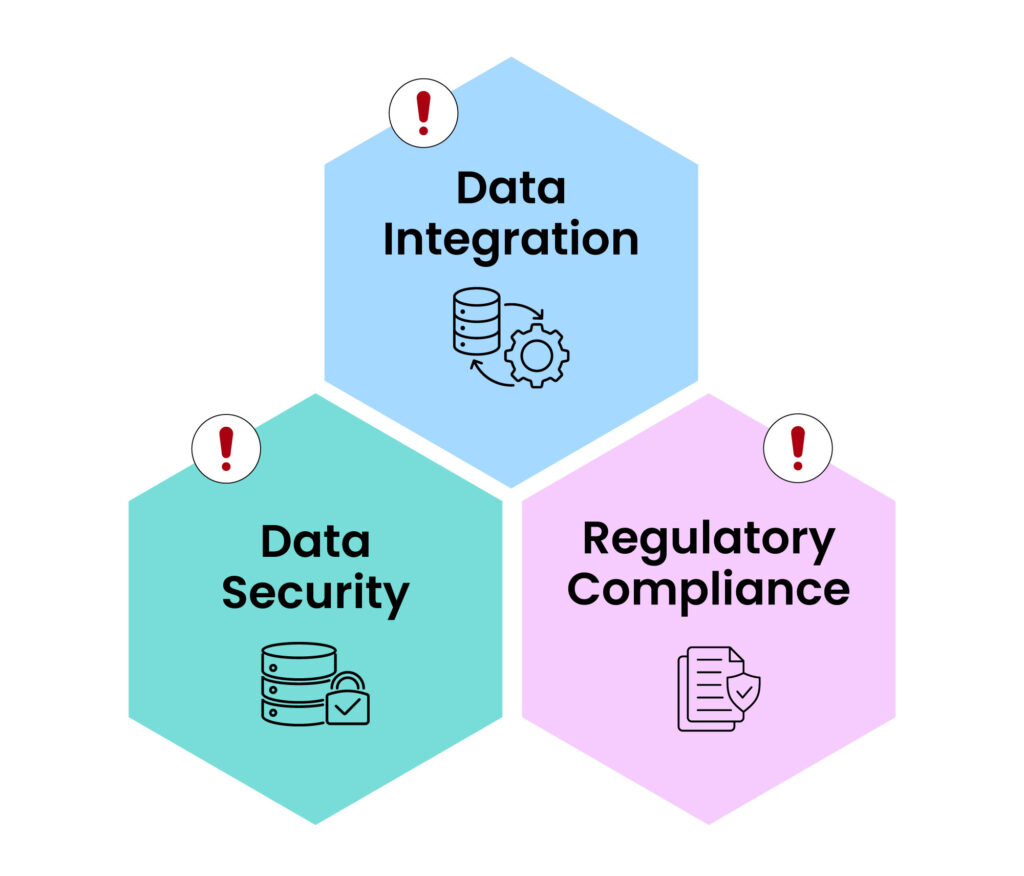
Figure 3: CDM Issues
1. Data integration:
Incorporating distinct data types, including proteomics and genomics, creates a significant challenge in data management. The intricacy and quantity of these data types involve specialized tools and proficiency to oversee efficiently. Successful data integration needs spirited systems and skill to make certain that entire data resources are consistent. Researchers can acquire a thorough knowledge of trial results, by ensuring that data from various resources is consistent and can be scrutinized collectively.
2. Regulatory compliance:
CDM need adherence to rigorous regulatory specifications, which can differ across areas. Although these criteria are proposed to guard patient security and confirm the dependability of trial outcomes, excluding they can likewise initiate considerable trials for researchers. Thorough knowledge of these guidelines and precise documentation is involved in compliance is being ensured. Researchers can make sure that their trials converge all mandatory requirements, through keeping up informed on the newest regulatory conditions.
3. Data security:
It is crucial to protect the privacy and concealment of clinical trial data. Along with the increase in amount of data collected during trials, the consequence of data violations and unofficial gain access to has moreover increased. CDM systems need to have robust safety measures to protect personal sensitive data. Such measures involve encryption, control of actions, and regular safety audits, safeguarding that the patient information is always protected.
Conclusion
Clinical Data Management is a crucial element of clinical research, by making sure that data poised during trials is precise, reliable, and prepared for analysis. By knowing the roles and responsibilities in the CDM, along with the challenges faced, stakeholders can better appreciate its significance in the drug development procedure. Successful CDM practices not only boost the value and reliability of trial data, but it also helps to the overall success of clinical research. Since the field remains to develop, staying notified about the newest developments and tools in CDM will be necessary for researchers and organizations peeking to improve their clinical trials.
Clinical Data Management & Analytics by Excelra
Excelra provides end-to-end Clinical Data Management (CDM) and analytics services designed to support drug development, regulatory submissions, and real-world evidence generation. At the core of their offering is expert clinical trial data curation and standardization, where data from trial registries like ClinicalTrials.gov and EUCTR, as well as scientific publications and conference proceedings, is extracted and organized into structured, machine-readable formats. This ensures consistency and usability across endpoints, interventions, populations, and outcomes — the PICO framework.
One of Excelra’s flagship resources in this space is the Clinical Trial Outcomes Database (CTOD), which houses curated data from over 100,000 clinical trials. This database is used extensively for competitive intelligence, trial benchmarking, and to inform evidence generation strategies for regulatory and payer submissions. In addition, Excelra supports Health Economics and Outcomes Research (HEOR) and Real-World Evidence (RWE) initiatives by extracting data from literature, claims, EMRs, and registries to model disease burden, patient journeys, cost-effectiveness, and treatment outcomes.
A key differentiator for Excelra is its integration of AI/ML tools and natural language processing (NLP) to scale up insight generation — identifying trends in trial outcomes, extracting predictive biomarkers, and suggesting optimal endpoints. They also build custom clinical ontologies and taxonomies, allowing for the semantic tagging of datasets, and the creation of knowledge graphs that link trials, interventions, and outcomes meaningfully.
In essence, Excelra’s clinical data management solutions blend deep domain expertise with scalable technology to streamline clinical research, improve trial design, and support commercial and regulatory strategies with real-world-ready evidence.

