Authors: Sidharth Shankar Jha, Mahendra Pal Singh, Prashanth Reddy Rikkala and Katyayni Vinnakota
Introduction
Biomarkers are indicators of normal physiological and pathogenic processes, and/or responses to therapeutic interventions. They are fundamentally required in drug development, facilitating response monitoring, dose optimization, and for identifying variations in drug response among patients. In general, biomarkers are of vast significance to biopharma, diagnostics, personalized medicine, and overall healthcare industry. Marketresearch.biz predicts that the biomarkers market valued at USD 58.6 billion in 2022 is expected to register a Compound Annual Growth Rate (CAGR) of 14.3% during the forecast period between 2023 to 2032. Biomarkers can be classified based on their nature, functional application. The BEST (Biomarkers, EndpointS, and other Tools) resource categorizes biomarkers into seven types as summarized in Figure 1.
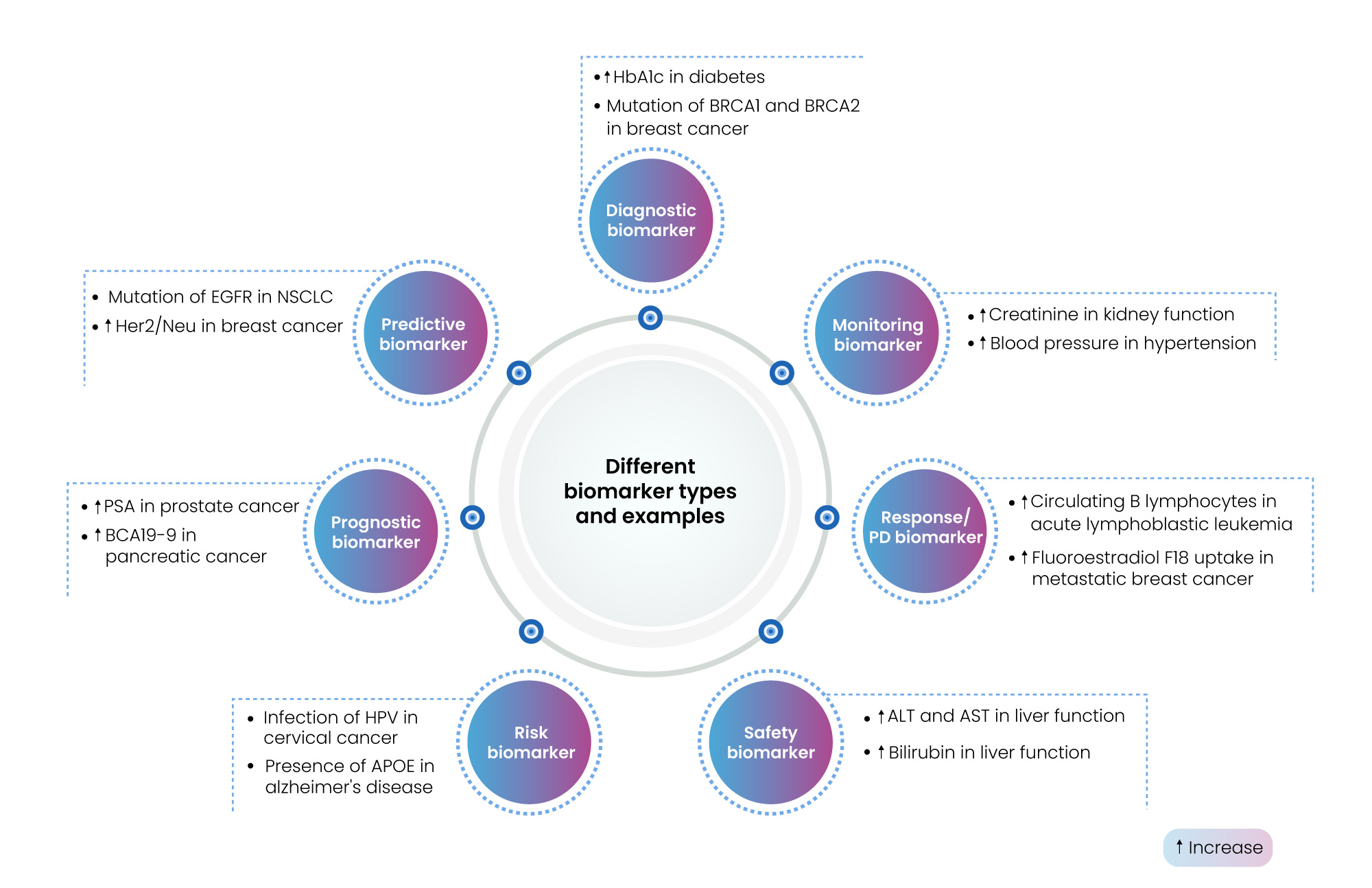
Figure 1: Different Biomarker types and examples
Biomarker landscape assessment is a comprehensive overview that identifies, analyses, and categorizes biomarkers in disease or drug development, focusing on their specificity, sensitivity, and utility. It is pivotal in healthcare and translational research. In the latter, the process validates biomarkers identified in preclinical studies for clinical relevance, aiding in the development of diagnostic tools, prognostic indicators, and safe and efficacious therapeutic interventions. Biomarkers play essential roles in various aspects of drug discovery and development like in validation of potential drug targets, and assessment of the mechanism of action (MoA). It plays a crucial role in clinical trials by facilitating patient stratification, endpoint selection, and treatment monitoring, thereby enhancing trial efficiency and success rates. The systematic assessment of the biomarker landscape is pivotal for the progression of personalized medicine. It involves identifying and understanding biomarkers linked to specific disease subtypes and therapeutic interventions. Moreover, biomarkers help to optimize patient outcomes while concurrently mitigating adverse effects.
Major challenges in biomarker landscape assessment:
Biomarker landscape assessment for a drug, a disease, or a target is associated with multiple challenges. Some of the major challenges are listed in Figure 2.
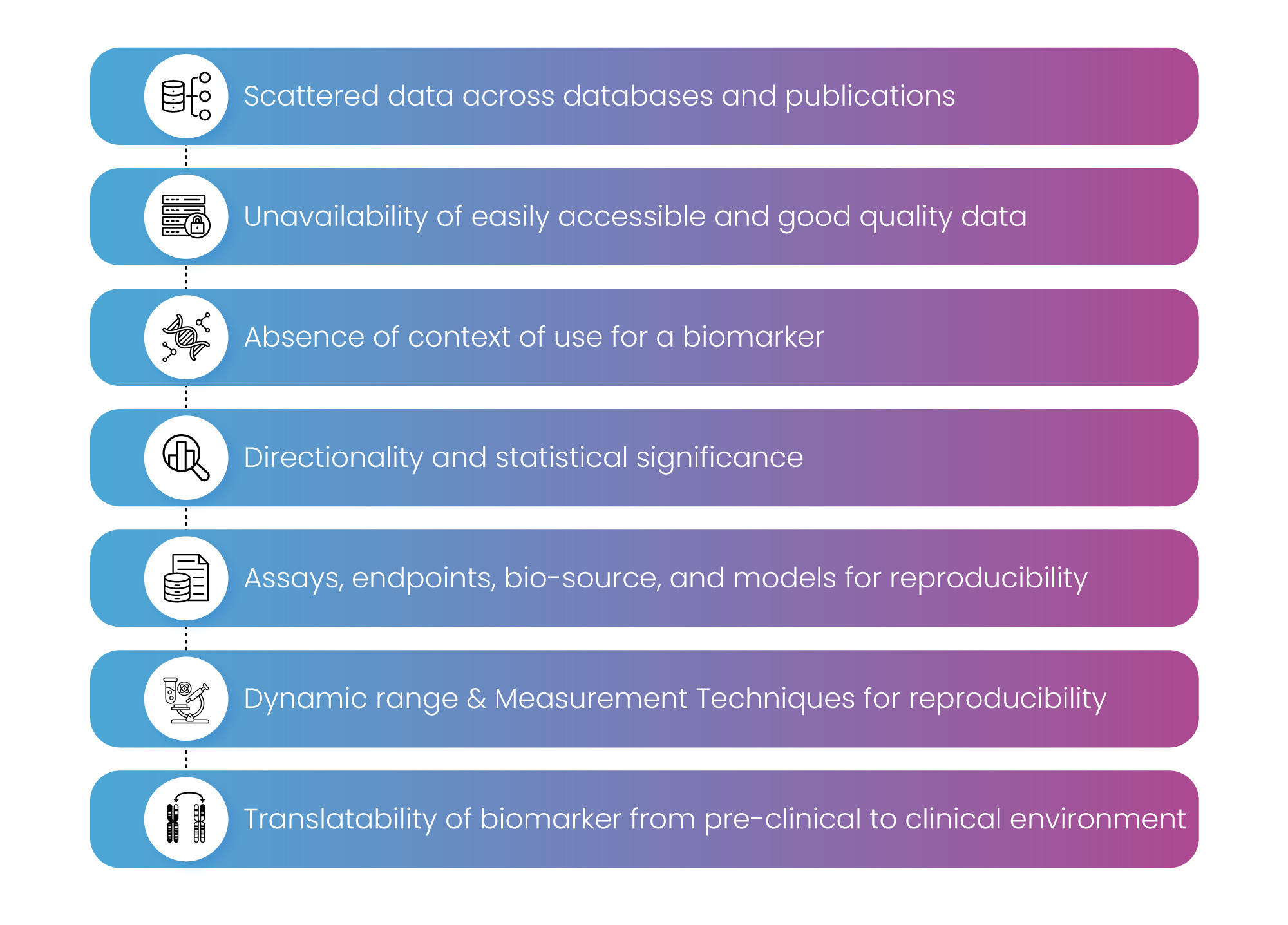
Figure 2: Major Challenges of Biomarker landscape assessment.
Excelra’s Approach to Biomarker Landscape assessment:
Excelra’s biomarker landscape assessment utilizes and integrates vital information from various systematic methodologies and resources, including proprietary databases, literature mining, clinical trial registries, computational analysis of data from high-throughput techniques, diagnostic imaging data, data analysis using statistical tools, and their application in building machine learning models for predicting and identifying suitable biomarker candidates as shown in Figure 3. The workflow helps interested stakeholders to make informed decisions pertaining to use of biomarkers in drug discovery and development programs.
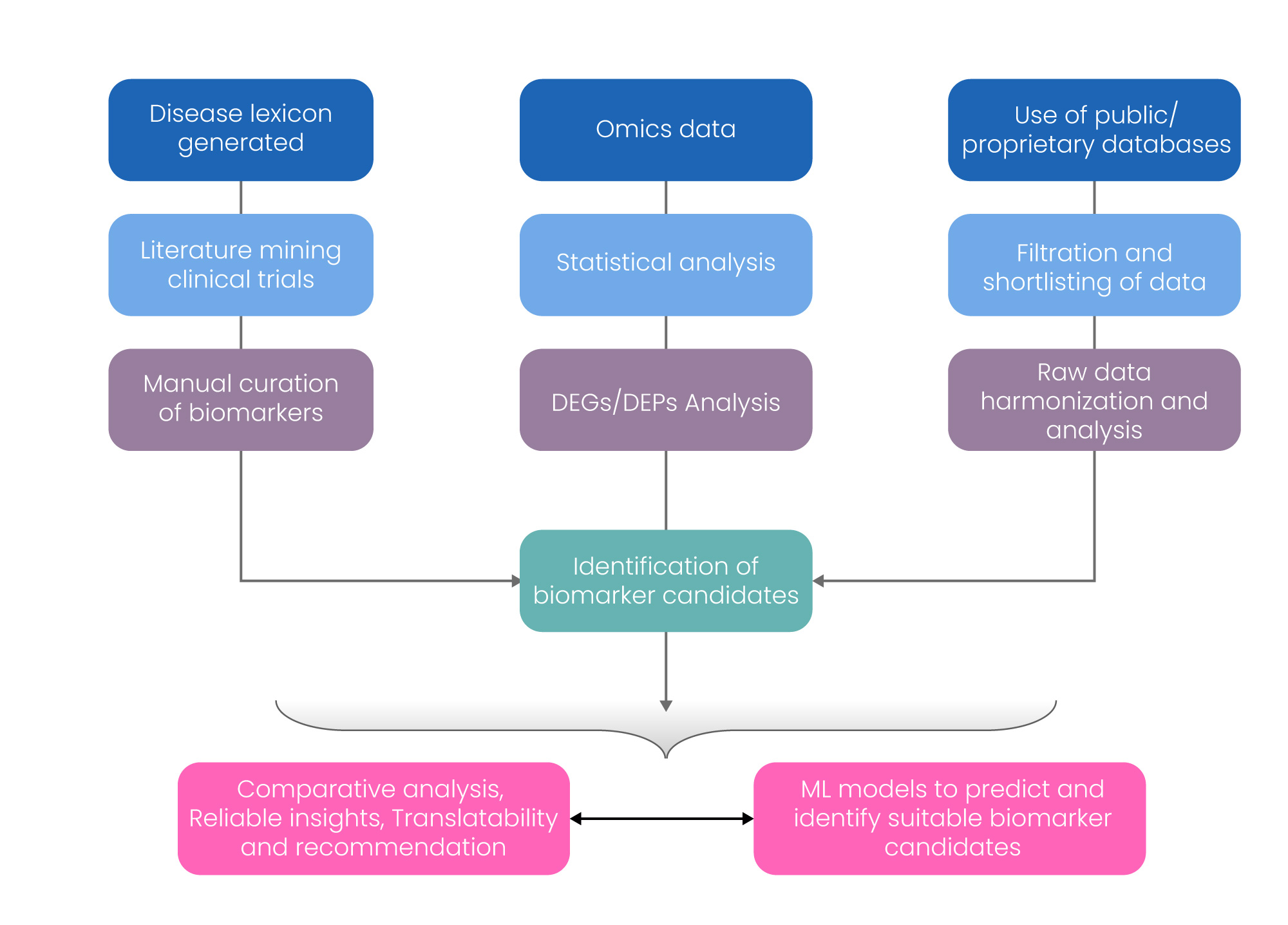
Figure 3: Excelra’s Approach of Biomarker Identification. DEGs- Differentially expressed Genes; DEPs- Differentially Expressed Proteins
Biomarker landscape assessment for Prostate cancer – a case study
Validation across diverse patient populations is crucial for clinical utility, while standardizing assay techniques and integrating biomarkers into guidelines is essential for widespread adoption. Addressing ethical and regulatory considerations is paramount. Our services offer a comprehensive profile, covering protein and gene aspects, leveraging a vast biomarker database to accelerate drug development for our partners. The landscape of prostate cancer biomarkers presents challenges, including the need for improved sensitivity and specificity for early detection and risk assessment (Figure 4).
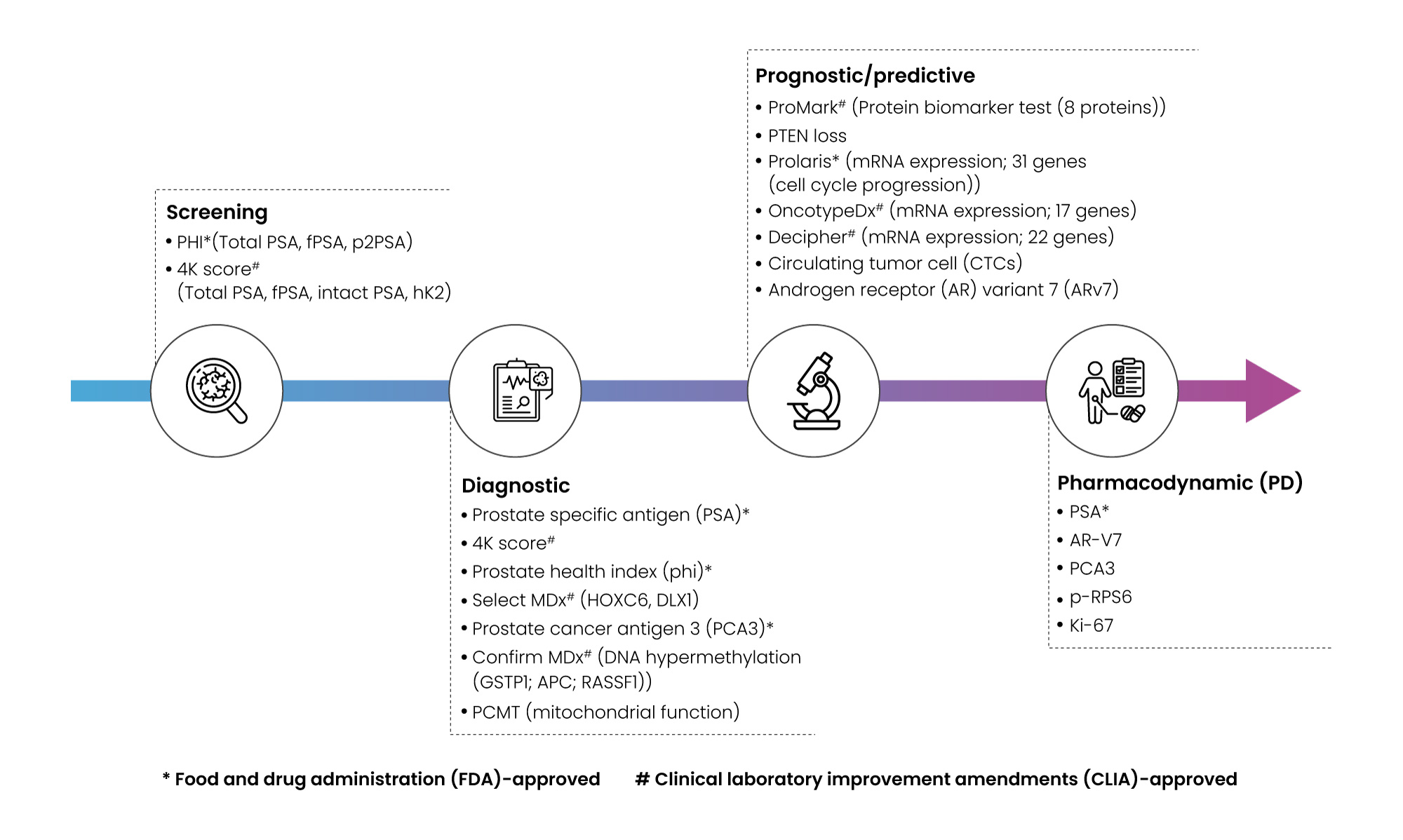
Figure 4: Prostate cancer biomarker landscape assessment.
Excelra’s USPs/Differentiators:
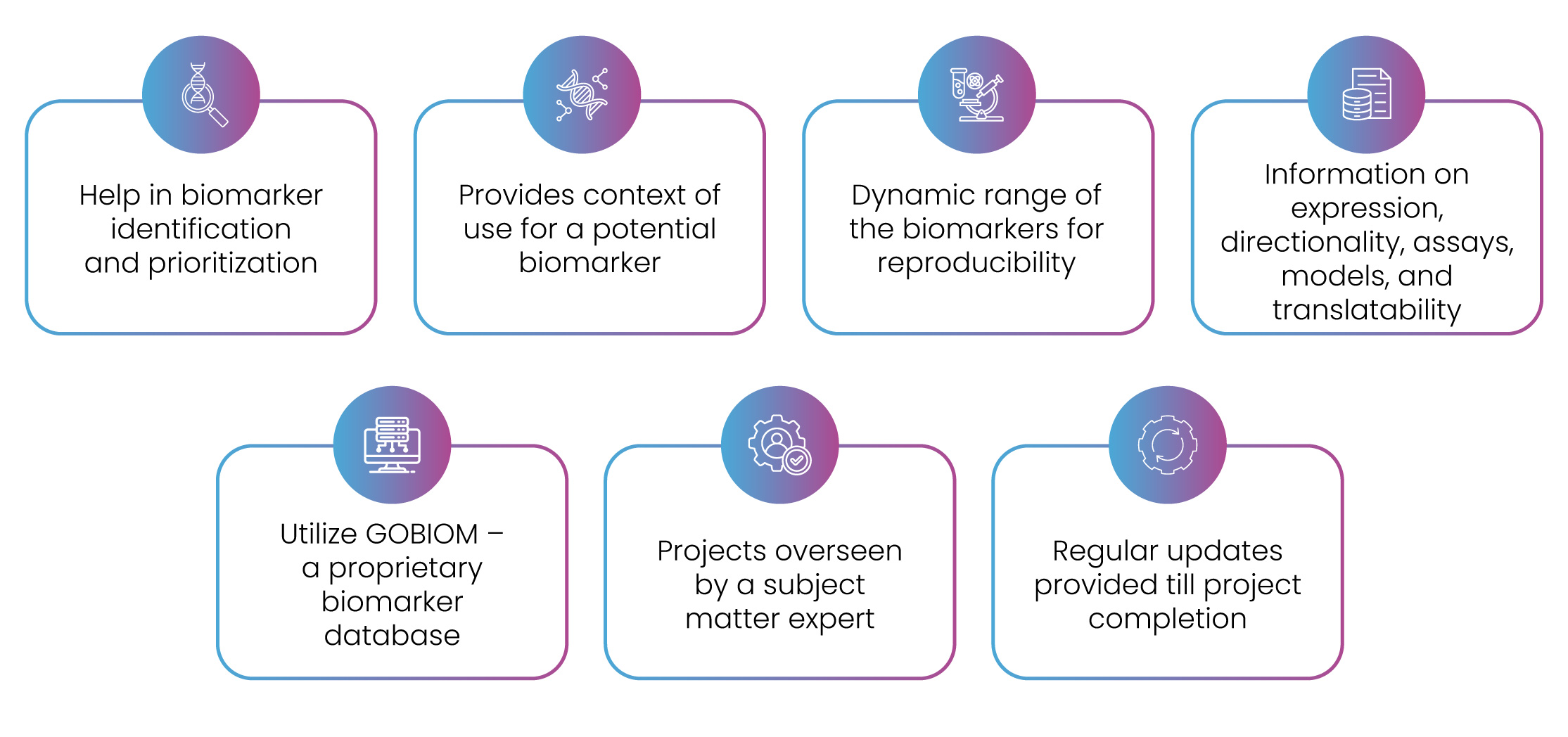
Figure 5: Excelra’s USP for biomarker landscape service.
Biomarkers play a crucial role in various aspects of healthcare, including disease diagnosis, prognosis, drug development, and monitoring therapeutic effects. Despite their immense potential, the conventional process of identifying biomarkers is often laborious and resource intensive. Both healthcare and pharmaceutical companies seek streamlined solutions to identify potential biomarkers and assess their translatability from pre-clinical models to clinical settings. Furthermore, the landscape of biomarker evaluation is undergoing a transformative shift with the integration of AI/ML models. These sophisticated algorithms enable the analysis of complex, high-throughput multi-omics, GWAS, and clinical data, revolutionizing biomarker assessment.
At Excelra, we offer tailored biomarker assessments designed to meet the unique requirements of our global Biotech and Pharma clients. Our comprehensive services cover specificity, sensitivity, and stability profiling (Figure 5). Leveraging our proprietary biomarker database and the expertise of our subject matter specialists, Excelra is well-positioned to support our partners in expediting their drug discovery and development endeavours.
That’s why you need more than just data. That’s why you need Excelra. Where data means more.


