Drug repurposing or repositioning is a promising approach for identifying new indications for approved or investigational (including clinically failed) drugs that have not been approved by FDA.
Drug repurposing evolved as an impeccable strategy because these drugs can bring medications to patients much faster and with less cost than that of developing new drugs. Safety and ADMET (absorption, distribution, metabolism, elimination, toxicity) of these drugs has already been tested in clinical trials for other indications.
COVID‐19 is an acute respiratory disease caused by the RNA virus SARS‐CoV‐2. The situation of the COVID‐19 pandemic is continuously evolving rigorously in more than 180 countries. Effective treatments are in urgent need, but currently, no drug with stable performance has been found for COVID‐19 hence, drug repurposing has evolved as a promising strategy for COVID‐19 treatment.
SARS-CoV-2 requires host cellular factors such as angiotensin I converting enzyme 2 (ACE2), transmembrane serine protease 2 (TMPRSS2) and furin for successful replication during infection. Systematic targeting of the SARS-CoV-2 interactome offers a novel strategy for effective drug repurposing for COVID-19.
Through affinity purification mass spectrometry, SARS-CoV-2 virus-host interactome shows 332 high-confidence protein-protein interactions between 26 viral and human proteins.
List of drugs that may be used against COVID-19
A comprehensive literature search to identify potential pharmacological agents that may be used against COVID-19 have yielded following 4 classes of drugs
- Drugs acting on viral replication
- Drugs acting on viral entry
- Drugs acting on cytokine release
- Drugs enhancing Immune response/endothelial dysfunction
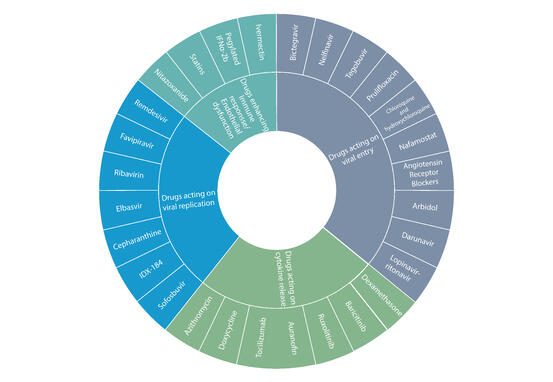
Figure 1: List of drugs being evaluated against COVID-19 Source: Drug repurposing approach to fight COVID-19
GOBIOM approach on drug repurposing for COVID‐19 treatment
GOBIOM’s COVID-19 Biomarker Database has been released in support of the ongoing global scientific efforts, aimed at developing safe and effective therapeutic options to treat the novel coronavirus disease.
The COVID-19 Biomarker Database provides data insights on repurposed drug and the indication for which drug is approved to treat, target (Biomarker) of the drug in pathology and its mode of action with clinical outcome of drug response. Database includes information on highest developmental status of the drugs that are in various ‘clinical, pre-clinical and experimental’ stages of drug discovery and development with direct access to scientific literature evidences.
OVERVIEW OF PROMINENT REPURPOSED DRUGS FOR COVID-19 IN GOBIOM DATABASE
Antiviral Drugs and Targets/Biomarkers
Remdesivir: A monophosphate prodrug of an active C-adenosine nucleoside triphosphate analogue, was originally used for the potential treatment of Ebola virus disease. Remdesivir significantly reduces the median recovery time to 11 days, compared with 15 days in the placebo group.
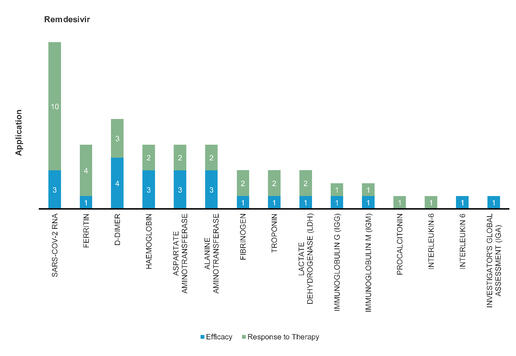
Figure 2: Pharmacodynamic/Response biomarkers of COVID-19 patients treated with Remdesivir
Favipiravir: Favipiravir significantly reduces virus clearance time and improves chest imaging with fewer adverse reactions. Favipiravir led to significantly accelerated relief of symptoms including pyrexia and cough. It is approved for Influenza.
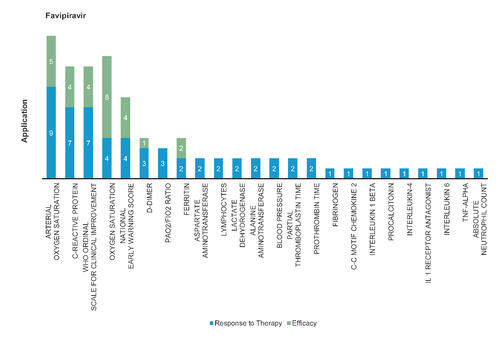
Figure 3: Pharmacodynamic/Response biomarkers of COVID-19 patients treated with Favipiravir
Ribavirin: Ribavirin mimics ATP and GTP to incorporate with RNA dependent RNA polymerase. Ribavirin efficacy increased in combination with IFN‐beta‐1b, lopinavir or ritonavir. Mainly used in treatment of Hepatitis C and RSV infection.
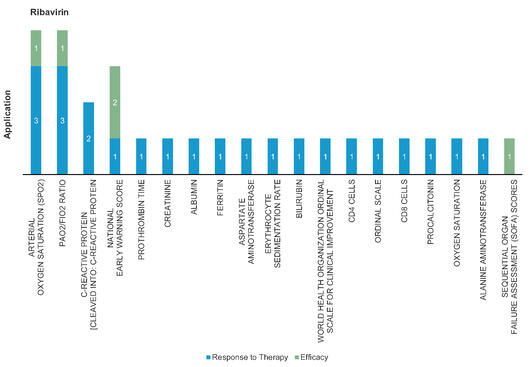
Figure 4: Pharmacodynamic/Response biomarkers of COVID-19 patients treated with Ribavirin
Virus Entry Inhibitors
Chloroquine: Chloroquine is a well-known antimalaria drug for many years. It disrupts virus‐receptor binding by interfering with glycosylation of the angiotensin‐converting enzyme 2 (ACE2). SARS-CoV-2 spike protein binding to ACE2 but also host gangliosides, and chloroquine interferes with this process by competing with the virus’s spike protein to bind to gangliosides. Chloroquine phosphate significantly decreased the disease duration compared to ritonavir‐lopinavir treatment.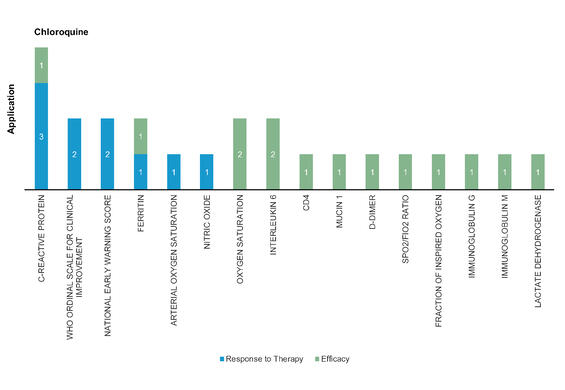
Figure 5: Pharmacodynamic/Response biomarkers of COVID-19 patients treated with Chloroquine
Hydroxychloroquine: Hydroxychloroquine is the hydroxylated form of chloroquine and shows similar antiviral mechanism. Hydroxychloroquine add‐on therapy to ritonavir‐lopinavir may have many potential adverse effects including cardiac, metabolic, and neurological symptoms, and so forth, and should be used with caution.
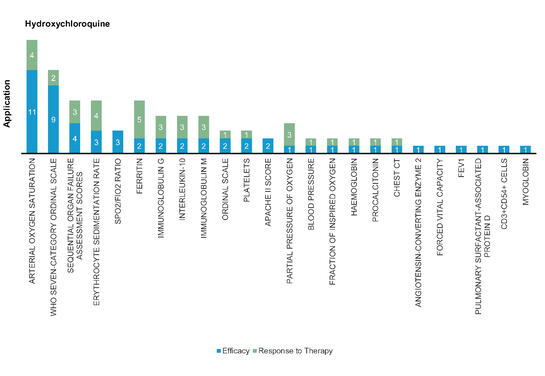
Figure 6: Pharmacodynamic/Response biomarkers of COVID-19 patients treated with Hydroxychloroquine
Non-virus Targeting Drugs
Tocilizumab: A monoclonal antibody which is an IL‐6 receptor antagonist mainly used for systemic juvenile idiopathic arthritis, polyarticular juvenile idiopathic arthritis and rheumatoid arthritis treatment. Severe or critical COVID‐19 infection showed that use of tocilizumab immediately repeated doses improved clinical outcomes.
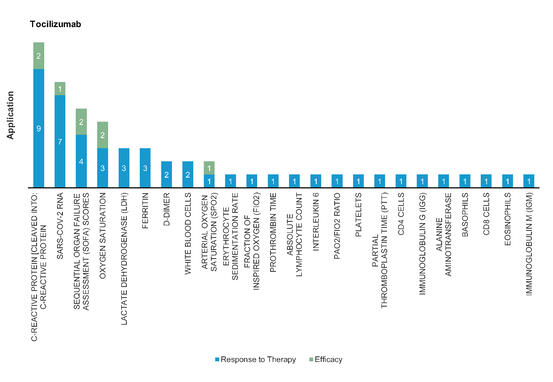
Figure 7: Pharmacodynamic/Response biomarkers of COVID-19 patients treated with Tocilizumab
Dexamethasone: An FDA approved synthetic corticosteroid and first‐line treatment for immune‐related complications by suppressing naïve T cell proliferation and differentiation in immune system. Decrease 28-day mortality rate by one-third in COVID-19 patients receiving invasive ventilation and one-fifth in patients receiving oxygen support.
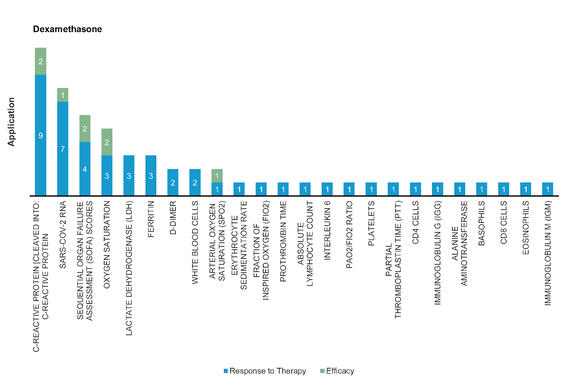
Figure 8: Pharmacodynamic/Response biomarkers of COVID-19 patients treated with Dexamethasone
Dapagliflozin: A sodium‐glucose cotransporter‐2 (SGLT2) inhibitor and is hypothesized to be able to prevent serious side effects caused by SARS‐CoV‐2 infection through preventing low PH in cells.
Figure 9: Pharmacodynamic/Response biomarkers of COVID-19 patients treated with Dapagliflozin
Anticoagulant treatment: Unfractionated heparin and low molecular weight heparin are mainly used to prevent or treat thrombosis. Low molecular weight heparin improves coagulation dysfunction in COVID-19 patients and exerts anti-inflammatory effects by reducing Interleukin 6 (IL-6) and increase the percentage of lymphocytes.
Conclusion
Excelra’s COVID-19 Biomarker Database provides mechanistic insights into SARS-COV-2 biology and disease pathogenesis with drug repurposing approaches which can help global biotech and pharma community to develop treatments for COVID-19.
Challenges
- Limited repository of drugs to be repurposed, owing to the high attrition rate in drug development and approval
- Repurposed drugs might have been optimized for a target, dosing, or tissue in the original indications
- Substantial investment is required towards the research and clinical trial programs for the repurposed drug as the drug efficacies and safety profiles for COVID-19 are not well-established
- Rapid clinical tests of existing antiviral, antimalarial, and immunomodulatory drugs have been done or are underway against COVID-19
- The presence of heterogeneous populations with different genetic backgrounds might also affect outcomes of clinical results


