To understand a drug’s effect on the body, investigations take place to understand the pharmacokinetic (PK), pharmacodynamic (PD), and toxicokinetic (TK) profiles of therapeutic target profiles.
Understanding these profiles in animals during preclinical trials and humans in clinical trials is mandatory in the successful progression of a drug toward regulatory approval. PK, PD, and TK investigations have improved significantly in recent years. However, there remain some fundamental questions that often delay commercial development, particularly in the PK arena. These questions are answered by the translation of PK data from humans to animals to humans, and from in vitro assets to in vivo readouts. The faster and more accurate these translations, the faster the delays are overcome.
PK refers to the biological processes determining absorption, distribution, metabolism, and excretion (ADME) of a potential treatment drug or drug in a living organism.
TK is closely related to PK and is largely reflective of the ADME of a molecule as it moves through the body of an organism. It’s defined as the generation of PK data via specifically designed supportive studies on the systemic exposure of a drug. The primary objective of TK is to describe the systemic exposure achieved in animals and its relationship to dose level and the time course of the toxicity study.
PD refers to drug action on the living organism, which includes not only the pharmacologic response, but also the duration and magnitude of drug response at an active site in an organism.
Explaining negative effects using quantifiable endpoints
The no-observed-adverse-effect-level (NOAEL) plays an important part in preclinical or non-clinical risk assessment. NOAEL is defined as “the highest experimental point that is without adverse effect.”1
Toxicologists provide a consensus professional opinion based on:
- The design of the study
- Drug indication
- Expected pharmacology or on-target benefits
- Spectrum of off-target or adverse effects
It’s important to note that NOAEL doesn’t address any risk interpretation based on toxicologically relevant effects. Nor does it reflect the progression of such effects in relation to the dose or duration of drug treatment.
Excelra’s preclinical toxicology report digitization (PTRD)
Given the importance of preclinical toxicology investigations, it’s clear that the data used in testing must be optimized and standardized before assessment. Inconsistencies in data may lead to inaccurate conclusions. Excelra’s PTRD solutions ensure there are no inconsistencies and that any conclusions can be confidently relied upon.
Of equal importance is the standardization and digitization of the reports that the testing produces. These reports are highly specialized data in themselves and require appropriate standardization before they can provide valuable insight. Whether legacy reports that require digitization and alignment, or freshly produced or sourced data, our PTRD pipeline accelerates the process, rapidly and accurately determining safety and efficacy.
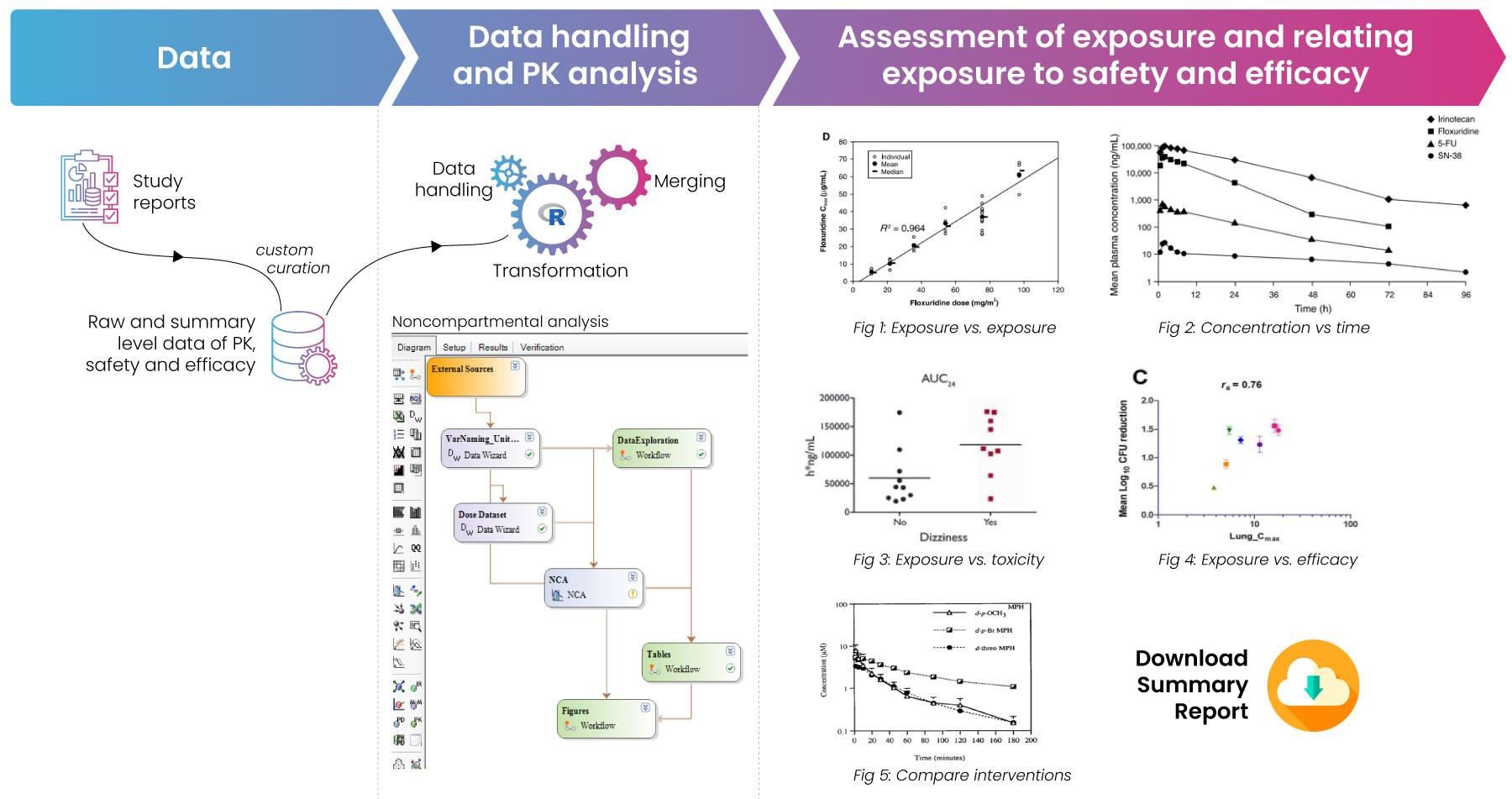
Fig 1: Process map for the creation of PTRD reports. The process is initiated with raw data curation based on customized variables. The data is then analyzed and the outputs are summarized visually.
Pharmacokinetics data
PK data analysis defines the relationship between the body’s exposure to a drug and the dosing regimen.
The concentration time curve determines the disposition and half-life of a drug. Published pharmacokinetics studies provide differences in the body’s drug exposure response due to different covariates or variables.
Data for safety studies / Toxicokinetics (TK)
The collection of TK data is a fundamental part of toxicity studies. It allows correlations to be drawn between the concentration of drugs and their metabolites in plasma or serum. It’s essential to the work of pathologists, toxicologists, pharmacokineticists, and clinical chemists.
Our team of data and research scientists specializes in creating customized preclinical toxicokinetic reports. We provide exceptional data capture and curation services to help you reach your objectives. We also develop analysis pipelines tailored to your variables and required metadata (Fig 2), and output in high-quality customized reports. A complete list of specific PK variables, descriptions, and standards is provided along with each report.
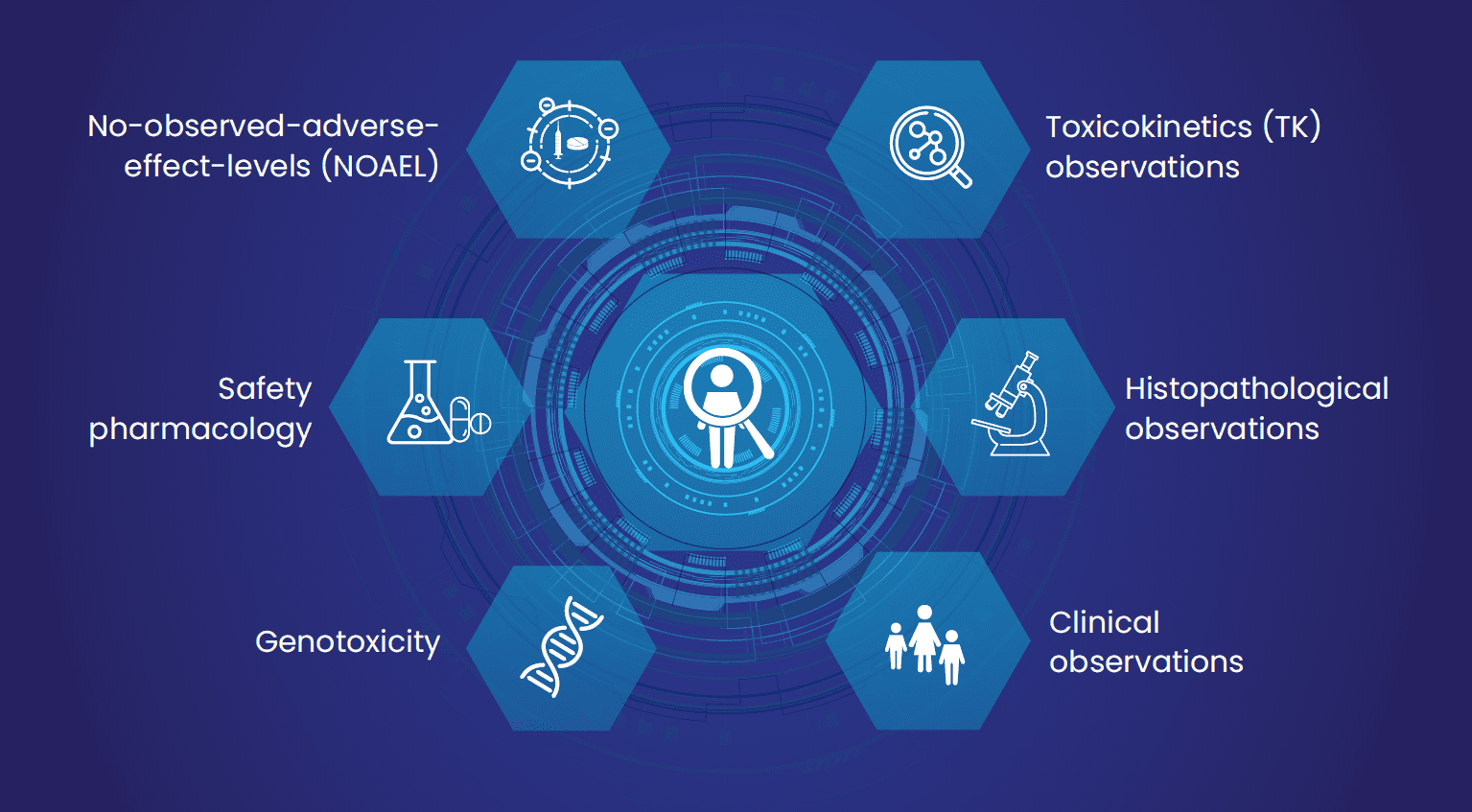
Fig 2: Summary of different variables captured by Excelra under the PTRD screening tree (custom curation-review and data analysis).
Case study: Structuring of unstructured preclinical data for a large pharma in the US, working in multiple therapeutic areas
Objective
To capture preclinical pharmacokinetic data from an investigator’s brochure (IB) provided by the client.
Process and deliverables (refer to Fig 1)
- Data model: Structured relational data model was prepared and delivered to the client and got approved. The output consisted of 9 sections (disposition, lab, histological, clinical, meta, Tk, safety, genotoxicity, and NOAEL) with 358 variables.
- Data: Unstructured data was extracted before rationalizing and digitizing for the client’s requirements. Information was extracted from text, tables, and digitized figures in Excel spreadsheets.
- Standards: The prepared enumeration list and standards document which is actively used during the curation process was approved by the client. The standards were followed throughout the curation process as appropriate for the uniqueness of the data.
Final deliverable
Our PTRD team developed and delivered a consolidated dataset based on 65 IB, which has helped the client move toward their objectives.
References
- https://www.nuventra.com/resources/blog/what-is-toxicokinetics/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3342576/
- https://pubmed.ncbi.nlm.nih.gov/15979222/
- https://www.europeanpharmaceuticalreview.com/news/166698/digital-transformation-will-improve-clinical-trial-process-say-experts/
- https://www.sciencedirect.com/science/article/pii/B9780128198698000355#!
- https://www.nature.com/articles/s41597-020-0455-1
- https://academic.oup.com/nar/article/49/D1/D1358/5957165
- https://pubmed.ncbi.nlm.nih.gov/17943658/
1 Dorato, M. A., & Engelhardt, J. A. (2005). The no-observed-adverse-effect-level in drug safety evaluations: use, issues, and definition(s). Regulatory toxicology and pharmacology : RTP, 42(3), 265–274. https://doi.org/10.1016/j.yrtph.2005.05.004