Model informed drug discovery and development (MID3) is a paradigm of data analytics in drug development which facilitates the development and application of various quantitative approaches like exposure-response, biological, and statistical models to characterize pre-clinical and clinical data. MID3 is usually aimed at improving the quality, efficiency and cost effectiveness of decision making in drug development. The knowledge and inferences from this quantitative framework help in making informed decisions for dosage optimization and provide supportive evidence for efficacy, clinical trial design, and informing policies. MID3 approach enables multidimensional integration of data across targets/mechanisms of actions, molecules/drugs, doses/regimens, indications, trial designs, endpoints, and patient characteristics/populations.1
Model based meta-analysis (MBMA)
Model based meta-analysis is an important quantitative toolkit in MID3. MBMA is different from conventional meta-analysis as MBMA helps to integrate concepts of pharmacology and biology with statistical concepts.2,3
MBMA serves a range of purposes such as:
- To increase the number of observations and their statistical power, if Individual trials are too small to give reliable answers
- To improve estimates of the effect size of an intervention or association
- To put any one trial into perspective by examining all similar trials by analyzing individual patient level data along with competitor summary level data
- To assess the generalizability of the conclusions to a more varied range of patients or treatments protocols by examining variability between trials and studies and performing subgroup analysis
- To resolve uncertainty when reports disagree
- To identify the need and planning for larger trials of studies, by identifying the gaps in evidence
- To answer questions not posed at the start of individual trials by finding answers to strengthen submissions on drug efficacy to licensing bodies such as the FDA
- To retrospectively identify the point in time when a treatment effect first
- reached conventional levels of statistical significance
- To assess determinants of publication bias
- To investigate side effects, often rather infrequent, of a treatment
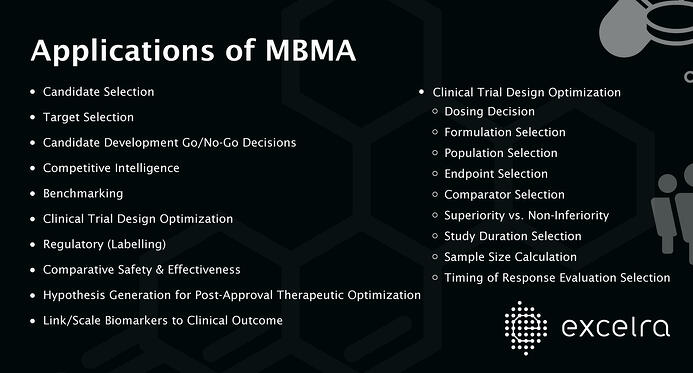
Use of MID3 approaches are increasing as the wide applications of them are established and are also gaining acceptance and interest from regulatory authorities.4 MBMA has proven applications in the complete life cycle of drug discovery and development from early development activities like candidate and target selection, early clinical phase decisions on candidate development and trial design to decisions during late clinical phase and post approval .1,3
Applications of MBMA
Identifying and preparing essential metadata available from the public domain of biomedical literature, specific to the analysis objective for MBMA requires voluminous efforts.
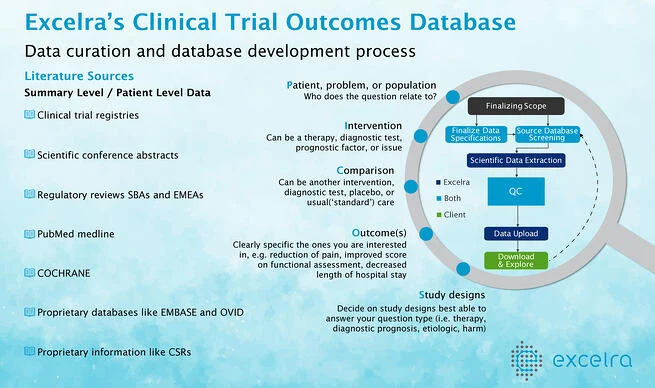
Analysis-ready datasets for MBMA
Excelra’s expert clinical data services team is helping the Quantitative Pharmacology and Pharmacometrics groups of pharmaceutical companies in building MBMA analysis specific datasets. This is performed with robust and customized, systematic literature review (SLR), following the industry-accepted PICOS methodology.
References
- Marshall SF, Good Practices in Model-Informed Drug Discovery and Development: Practice, Application, and Documentation. CPT Pharmacometrics Syst. Pharmacol. (2016) 5, 93–122
- Fagard RH, Advantages and disadvantages of the meta-analysis approach. J Hypertens Suppl. 1996 Sep;14(2):S9-12; discussion S13
- Upreti VV, Model-Based Meta-Analysis: Optimizing Research, Development, and Utilization of Therapeutics Using the Totality of Evidence. Clin Pharmacol Ther. 2019 Nov;106(5):981-992
- https://www.fda.gov/drugs/development-resources/model-informed-drug-development-pilot-program
- Demin I, Longitudinal model-based meta-analysis in rheumatoid arthritis: an application toward model-based drug development. Clin Pharmacol Ther. 2012 Sep;92(3):352-9


