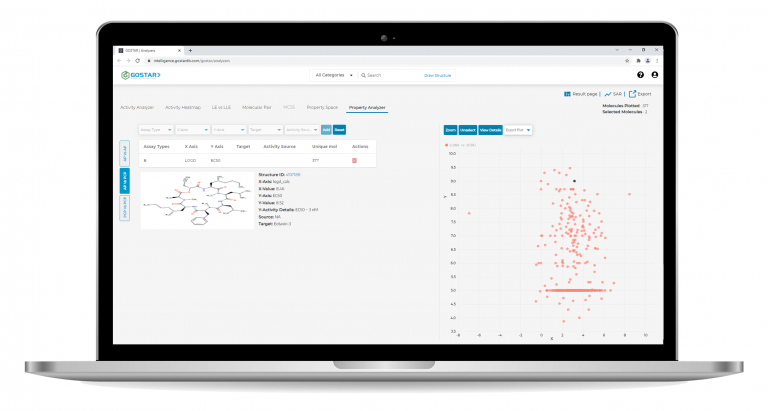
Interactive Property Space Exploration
Lipophilicity plays a significant role in small molecule drug design and discovery. A partition coefficient, logP, can be used to describe the lipophilicity of an organic compound. It is expressed as the ratio of the unionized compound’s concentration in the organic and aqueous phases at equilibrium. The distribution of species in compounds containing ionizable groups is influenced by pH and the lipophilicity of a molecule is affected by its ionization state. As a result, the distribution coefficient (logD) of a compound is defined, which considers the dissociation of weak acids and bases. In aqueous conditions, highly lipophilic substances are often less soluble. Lipophilic compounds, on the other hand, may have good solubility in oils and lipids, making them good candidates for lipid-based formulations.
Lipophilicity influences potency, selectivity, permeability, absorption, distribution, metabolism, excretion, and toxicity (ADMET) properties. High lipophilicity, with logP greater than five, is associated with limited solubility, increased clearance, and poor oral absorption. Furthermore, highly lipophilic drugs have a predisposition for interacting with hydrophobic targets other than the primary target, thereby enhancing promiscuity and toxicity. Low lipophilicity can reduce permeability and potency, resulting in lower bioavailability and overall efficacy. Compounds with logP greater than one or less than four are thought to have better physicochemical and ADME properties for oral drugs.
Lipophilicity is often regarded as a key indicator of potential promiscuity, with many property–promiscuity studies indicating that drug promiscuity rises with the increase in lipophilicity. This tendency is concerning since increasing a molecule’s lipophilicity can improve its efficacy at the primary target; however, this can be counterbalanced by an increase in off-target promiscuity [2]. Lipophilicity is a key element in determining a drug’s affinity for protein targets and in modulating ADMET characteristics. As a result, the combination of high target potency and high lipophilicity may increase the likelihood of ADMET-related attrition.
Therefore, medicinal chemistry optimization needs to be balanced and multidimensional. GOSTAR® empowers medicinal chemists to efficiently explore the property space against a variety of bioactivity endpoints.
References
1. Gao Y, Gesenberg C, Zheng W. Oral Formulations for Preclinical Studies: Principle, Design, and Development Considerations, Developing Solid Oral Dosage Forms (Second Edition), Academic Press. 2017, 455-495.
2. Armstrong D, Li S, Frieauff W, Martus H.J, Reilly J, Mikhailov D, Whitebread S, Urban L. Predictive Toxicology: Latest Scientific Developments and Their Application in Safety Assessment, Comprehensive Medicinal Chemistry III, Elsevier. 2017, 94-115.
If you are new to GOSTAR® and want to power your drug discovery with gold-standard data, request a demo now.


