“The industry is taking a more data-driven and strategic approach to Drug Repurposing.”
Dr. Nandu Gattu – Senior Vice President; Pharma Analytics, Excelra
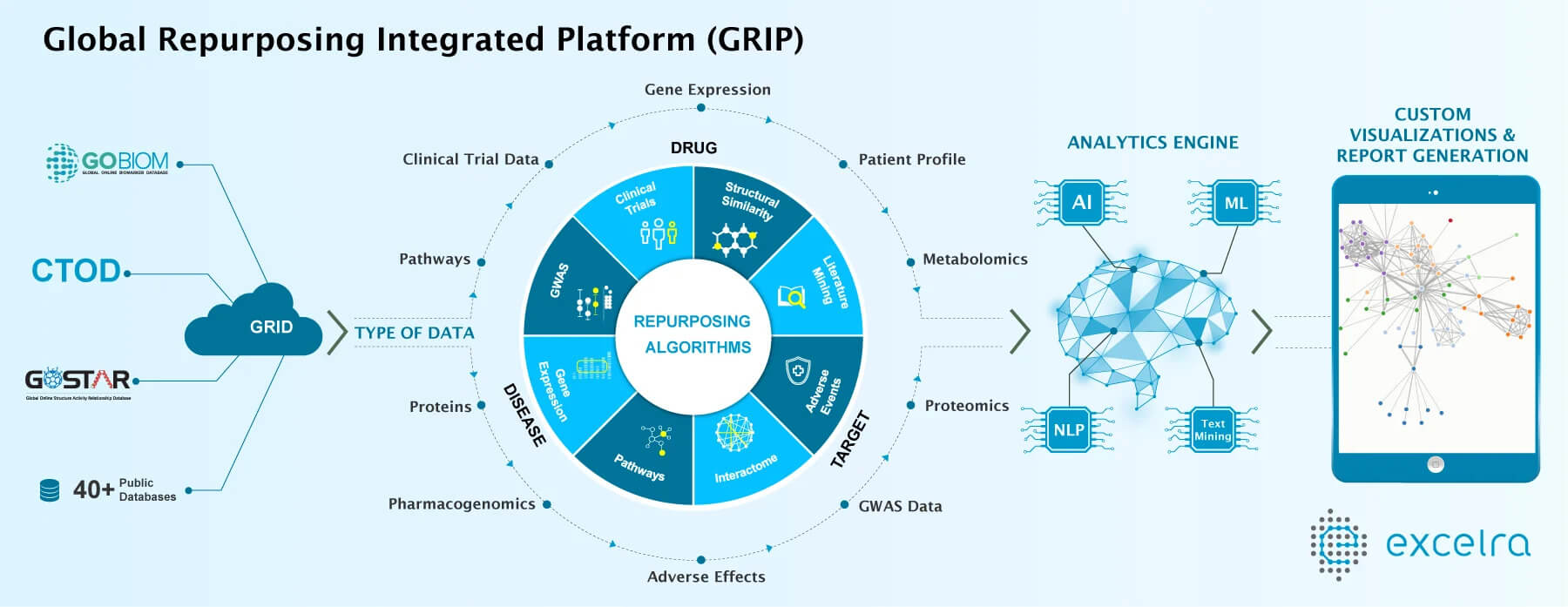
“Pharma is taking a more of a data-driven and strategic approach to Drug Repurposing,” says Nandu Gattu, Ph.D., Senior Vice President, Pharma Analytics and Drug Repurposing at Excelra, a data and analytics company. “Instead of waiting for serendipity driven drug repurposing, companies are evaluating the alternative indications of the drug considering the drug target, what pathways the particular drug is up-regulating and down-regulating; understanding what similar drugs are available; and examining current clinical trials that are being conducted.”
A strategy should include a review of the entire pharmacology, biology, toxicology, clinical trial information, genetics, genomics, and chemistry of that drug in a 360-degree manner, he says. Bringing all this evidence together creates a faster, smoother pathway to finding the science to a new indication. “We put together the information layer by layer, so at the end of the day, we have a very good scientific justification to make a recommendation,” Dr. Gattu says
Excelra recently signed on with Japanese pharmaceutical company Maruho to help the drug maker discover and develop novel therapeutic hypotheses in dermatology, and with Keio University School of Medicine in Japan to explore new therapeutics for a rare indication.
Excelra has a database of about 9 million structure-activity relationship compounds collected over the last 18 years from scientific publications and journals. “It is very useful for us to understand the similar compounds and extrapolate the information,” he says. “But there are two things necessary for all this analysis to be successful: algorithm and data.”
Dr. Gattu attributes the accessibility and the availability of more clinical trial data as a plus for driving a repurposing strategy. Companies no longer have to rely on their own proprietary data. There are myriad public data banks that researchers now have access to, and the computational tools in today’s market provide a way to parse through big data more easily. “There is a ton of a genomic and genetic-oriented data available in the public arena that we use on a regular basis,” Dr. Gattu says. “At the same time, there are quite a number of people opening up their clinical trial data, so this information is also being used.”
Data-driven approaches also include literature mining, which is now possible through text mining and NLP capabilities. This enables mining unstructured articles and receiving output in a structured manner. “This makes it easy to understand what’s going on, so we can connect A to B and B to C,” Dr, Gattu says. “There is always an opportunity for us to link between a drug and new diseases using this data-driven approach.” According to Dr. Gattu, repurposing will always be an opportunity for pharma companies for multiple reasons: drying pipelines, lack of new molecular entities being discovered, new technologies, and more data that provide the setting for repurposing. “New technologies are evolving, but at the same time, pipelines are not improving for individual pharma companies,” he says. “So, in my opinion, drug repurposing is going to be used even after a drug is on the market for 10 years because the more real world evidence that is available will help clarify the picture.”


