The ongoing COVID-19 pandemic is continuing to spread rapidly across the globe. As of 30th June 2020, the World Health Organization (WHO) has reported over 10 million cases and half a million deaths across 188 countries. Current clinical research is focused on accelerating the development of drugs and vaccines for treatment of SARS-CoV-2 infection. In this scenario, identification and selection of the right biomarkers is of paramount importance and plays a critical role for optimizing clinical trial design and successful drug development. It is quite cumbersome to find the biomarker data which is often scattered across public literature and considerable efforts go into data annotation from numerous disparate and dispersed sources.
Excelra’s COVID-19 Biomarker Database is an ‘Open-Access’ resource which is excerpted from our GOBIOM platform – the world’s largest biomarker intelligence database. COVID-19 Biomarker Database is a compilation of manually curated biomarkers from published clinical trials, evaluating potential drugs or biologics for the treatment of SARS-CoV-2. The database additionally includes information on FDA-NIH recommended BEST (Biomarkers, EndpointS and other Tools) classification of biomarkers, supported with direct links to the referenced literature. In the following sections, we present crucial statistics on the global COVID-19 clinical trial landscape with data and visualizations from the COVID-19 Biomarker Database. cumbersome to find the biomarker data which is often scattered across public literature and considerable efforts go into data annotation from numerous disparate and dispersed sources.
Clinical Trials Analysis
Clinical Trials Characteristics
Out of the 2351 registered clinical trials on COVID-19, 785 clinical trials with biomarker information were included in the COVID-19 biomarker database. The included clinical trials comprised of 627 (80%) interventional studies and 158 (20%) observational studies.
Biomarkers
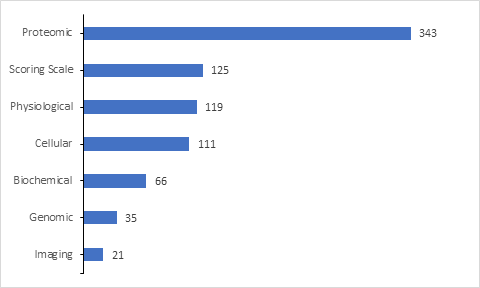
Of the 785 clinical trials with biomarker information which are included in the database, majority biomarkers are proteomic followed by Scoring scale, Physiological, Cellular, Biochemical, Genomic and imaging biomarkers.
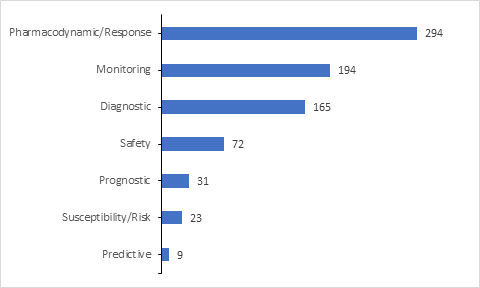
Majority of the biomarkers included in the trials are utilized for assessing the Pharmacodynamic/Response for the drugs under investigation.
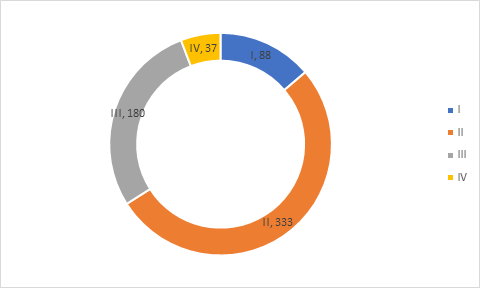
Clinical Trial Phase
Analysis of clinical trials in COVID-19 biomarker database suggest that majority of trials are in Phase II (52%) followed by phase III (28%), Phase I (14%) and Phase IV (6%).
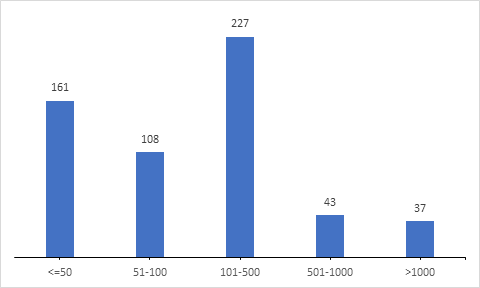
Study Population Size
Further analysis on the sample size included in the clinical trials reveals that majority of the trials (28%) recruit 100-500 patients in the clinical trials.
Drugs/Therapies Investigated
Overall, 535 clinical trials were registered for testing the therapeutic benefits of potential drugs/vaccines, including 133 (25%) clinical trials for monotherapy and 404 (75%) clinical trials for combination therapy.
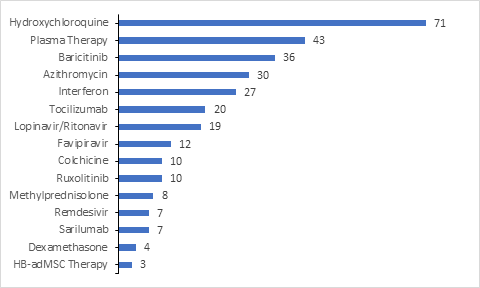
Research Landscape
Numerous clinical trials have been registered by the Industry/Academia/Research institutes in clinicaltrials.gov for evaluating Pharmacodynamic/Response for the drugs under investigation.
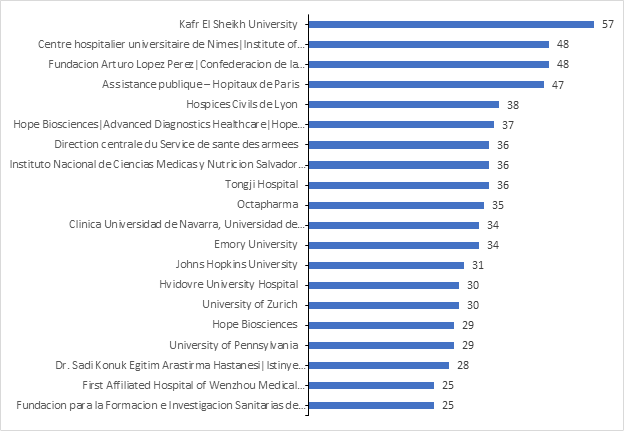
Below are the top contributors by no. of studies
Conclusion
Analysis of the data in COVID-19 biomarker database shows that the primary focus of the current clinical trial research is to evaluate Pharmacodynamic/Response for the drugs under investigation. Of the studies evaluating Pharmacodynamic/Response for the drugs, majority studies (75%) have included combination drug therapies. Given that most of the clinical trials (28%) include 101-500 patients in the study, many of these studies are likely to provide evidence on efficacy and safety of the investigated therapy.
It is noteworthy that our analysis was limited to clinical trials registered in clinicaltrials.gov.
Excelra’s COVID-19 Biomarker Database has been released in support of the ongoing global scientific efforts, aimed at developing safe and effective therapeutic options to treat the novel coronavirus disease.


