Overview
Excelra partnered with a leading biotech company to modernize its Genomic Variant Analysis workflow. By replacing manual, Excel-based processes with a scalable web platform, the solution reduced manual effort by 70%, improved turnaround time by 50%, and ensured 100% data integrity and zero compliance gaps. This transformation enabled higher diagnostic accuracy, better team collaboration, and faster, audit-ready operations.
Our client
A leading biotech company specializing in clinical genetic testing and Next-Generation Sequencing (NGS). Known for delivering actionable insights for patient care, the client needed a modern, scalable solution to improve the accuracy, compliance, and throughput of its diagnostics workflow.

Client’s challenge
- Relied on error-prone, Excel-based genomic variant analysis workflows.
- Faced high manual effort and limited scalability.
- Lacked end-to-end audit trails and traceability.
- Experienced delays due to fragmented, manual annotation and review.
Client’s goals
- Eliminate manual inefficiencies via automation.
- Improve data accuracy and reduce human error.
- Ensure full auditability and compliance readiness.
- Enable collaboration and faster decision-making via a unified platform.
Our approach
Excelra took a consultative and phased approach to solution delivery, ensuring that both technical and clinical considerations were addressed:
- Discovery & Workflow Mapping: Conducted stakeholder interviews to map current workflows and identify key pain points.
- Requirements Consolidation: Captured functional, data, and compliance needs to define a future-ready blueprint.
- Logic Extraction & Translation: Converted Excel macros and rules into scalable backend logic for the web application.
- Phased Development & Testing: Rolled out features incrementally for faster feedback and smooth adoption.
- User Enablement & Change Management: Delivered training, manuals, and live support for seamless onboarding.
Our solution
Building a scalable, audit-ready diagnostics platform
To address the client’s challenges and goals, we implemented comprehensive three-tiered architecture designed to streamline the genomic variant analysis workflow, enhance data accuracy, and improve operational efficiency. The solution included the following key components:
Tier 1: Web application
- Centralized variant review, project tracking, and collaboration.
- Real-time auditability and submission status visibility.
- Intuitive interface for faster variant annotation and approval.
Tier 2: Data ingestion & curation
- Automated JSON ingestion to eliminate manual entry.
- Applied client-defined scripts for preprocessing and validation.
- Used Apache Event Hub for audit trail logging and monitoring.
Tier 3: MS SQL database
- Structured SNV/CNV and variant library storage.
- Full audit trail capture for every workflow step.
- Fast, reliable access to curated diagnostic data.
Implementation highlights
- Mapped and customized workflows through collaborative sessions.
- Deployed incrementally to minimize disruption.
- Trained users with tailored modules to drive adoption.
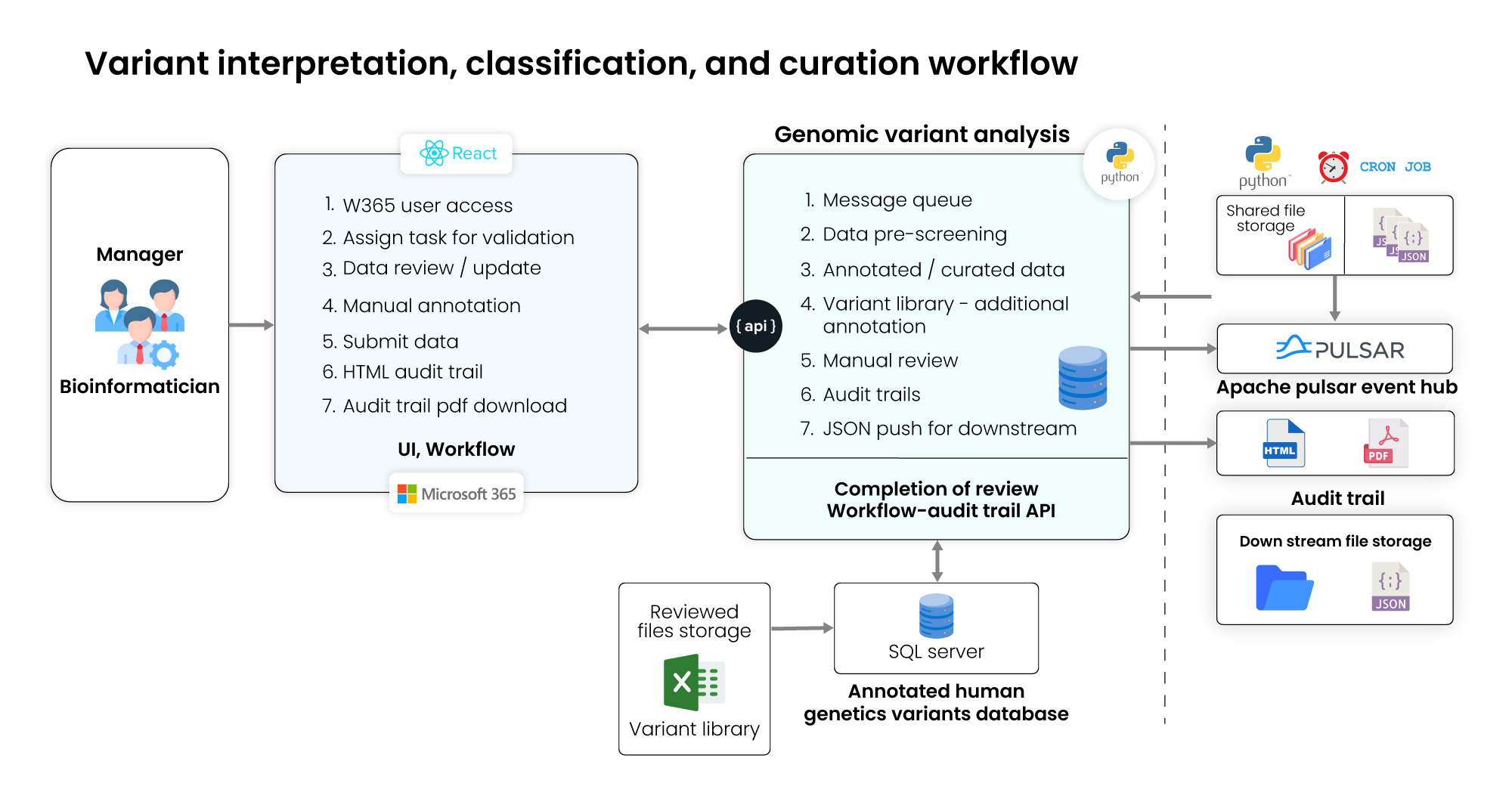
Figure: Technical architecture
Impact and results
- 70% reduction in manual interventions
- 50% faster variant classification turnaround
- 100% data integrity through SQL-based validation
- 0 compliance gaps with real-time auditability
Conclusion
Collaboration improved dramatically as all stakeholders worked within a unified platform, setting a new standard for operational efficiency, accuracy, and scalability.

