Overview
A leading US-based animal health company aimed to develop stapled peptide antagonists targeting a pleiotropic cytokine receptor crucial for immune modulation in livestock. With no structural data and poor peptide stability, the challenge was significant. Excelra applied a simulation-led approach, combining molecular modeling, MD simulations, and peptide engineering to design a library of structurally robust peptides. The peptides showed high binding affinity, cross-species efficacy, and improved stability. This strategy accelerated discovery timelines by up to 12 months, cut R&D costs by 30–50%, and increased hit rates threefold—delivering a scalable, species-relevant peptide discovery platform for next-generation veterinary immunotherapies.
Our client
A prominent US-based animal health organization recognized for its leadership in developing innovative therapeutic solutions for livestock—approached Excelra with a strategic objective. Their goal was to discover novel peptide-based antagonists targeting a cytokine receptor implicated in immune modulation and chronic infections in food-producing animals. With a strong focus on advancing next-generation veterinary biologics, the client sought to leverage molecular and structural insights to design robust, stable peptide therapies capable of disrupting cytokine-receptor signalling. Their broader vision centered on improving animal health outcomes by addressing persistent infectious challenges through targeted immunomodulation, using precision-designed peptides that are both efficacious and well-tolerated in livestock systems.

Client’s challenge
A major US-based animal health company wanted to develop peptide-based antagonists targeting a pleiotropic cytokine receptor known to play a central role in immune modulation and chronic infections in food-producing animals. The cytokine’s dual role in driving immune responses and offering host protection made it an attractive therapeutic target. However, the client faced significant challenges at the outset of the program like:
- Complete lack of experimentally derived structural data for the cytokine, making it difficult to understand the molecular basis of ligand-receptor interactions or to guide rational design efforts. Because, in the absence of structural data, critical information about binding interfaces, conformational flexibility, and key interaction residues remains unknown, severely limiting the ability to design targeted and effective peptide antagonists.
- Poor structural stability, particularly helicity loss under physiological conditions, which posed major concerns for in vivo efficacy and suitability in livestock.
Client’s goals
The client required a solution that would enable rapid, data-driven discovery, produce structurally robust and functionally validated peptide candidates, and ensure species relevance and translational potential for veterinary use. To address these challenges, the client leveraged Excelra’s expertise in molecular modelling, computational biology, and peptide engineering to build a reliable discovery framework and deliver a high-quality library of novel peptide antagonists.
Our approach
We implemented a stepwise, structure-guided discovery workflow that relied on advanced molecular modelling and simulation to overcome data gaps and stability concerns.
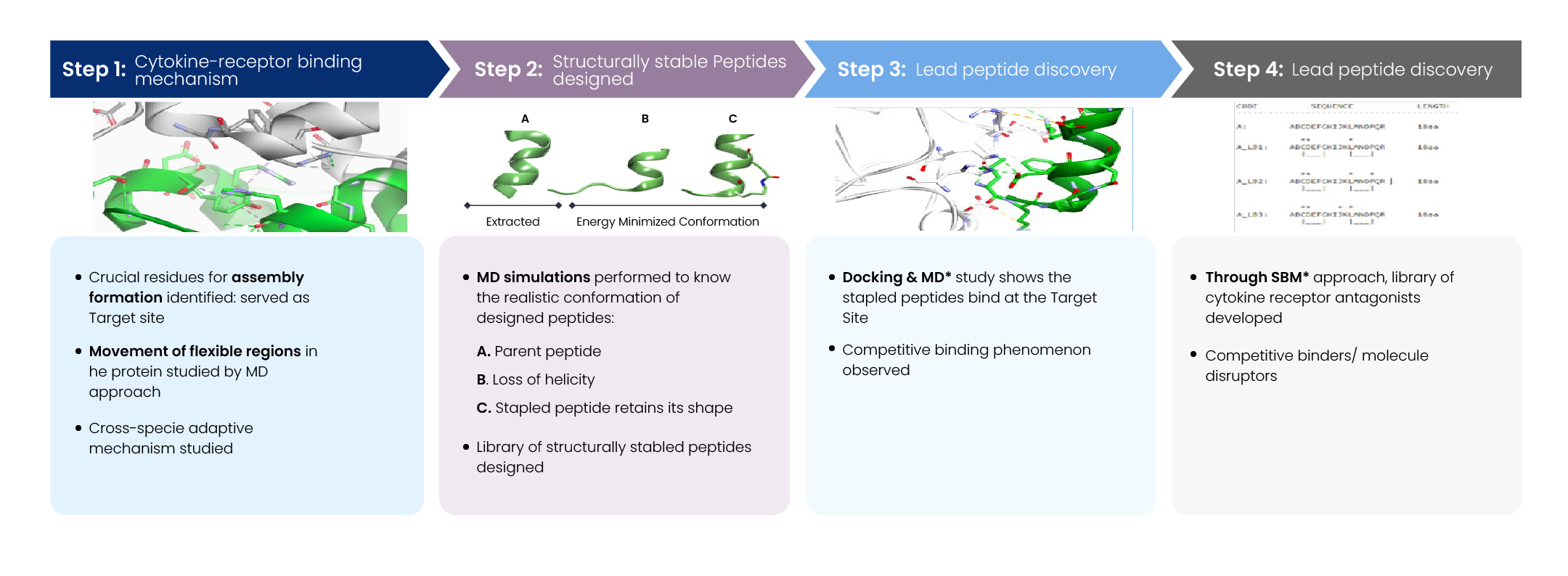
Step 1: Understanding Cytokine-Receptor Binding
To lay the foundation for rational design, we began by dissecting the molecular basis of cytokine-receptor interactions:
- Identification of Critical Binding Residues: We used available structural data (crystal/NMR) and sequence alignments to pinpoint residues essential for stable complex formation between the cytokine and its receptor.
- Molecular Dynamics (MD) Simulations: These simulations were instrumental in visualizing the conformational flexibility of binding regions. We particularly focused on loop regions and transient interfaces that contribute to binding specificity and adaptability.
- Cross-Species Comparative Analysis: To assess evolutionary conservation and species-specific variability, we compared cytokine-receptor interfaces across multiple orthologs. This allowed us to understand key adaptive residues and conserved hot spots relevant for antagonist design.
Step 2: Designing Structurally Stable Peptides
Building on structural insights, we moved toward stabilizing the biologically active conformation of the peptide:
- Parent Peptide Modelling and Assessment: The native peptide, derived from a key interaction motif, was modelled. MD simulations revealed a tendency for helicity loss in aqueous environments, indicating instability.
- Stapled Peptide Engineering: To counteract structural degradation, we introduced hydrocarbon staples and other helix-stabilizing modifications. These changes preserved the α-helical conformation critical for receptor engagement.
- Peptide Library Generation: A focused library of conformationally constrained analogs was created. Each design was subjected to MD validation to confirm helicity, compactness, and solvent exposure of key residues.
Step 3: Lead Peptide Discovery
With a structurally robust peptide pool in hand, we prioritized candidates with high binding affinity and desired functional properties:
- Docking and MD Refinement: Using structure-based docking followed by MD simulations, we screened for peptides that could effectively occupy the cytokine-binding site on the receptor.
- Competitive Binding Analysis: Several stapled peptides demonstrated the ability to competitively inhibit cytokine binding, suggesting potential antagonistic behavior.
- Lead Prioritization: Peptides were ranked based on binding affinity, structural stability, and disruption potential. The top candidates moved forward for experimental validation.
Step 4: Building a Novel Peptide Library
The final step focused on expanding the therapeutic potential by diversifying peptide scaffolds:
- Structure-Based Modelling (SBM): Leveraging high-resolution interaction data, we designed novel peptide scaffolds optimized for binding the cytokine-receptor interface with antagonistic intent.
- Identification of Functional Disruptors: Through iterative design and simulation, we identified peptides that not only bound competitively but also disrupted downstream signaling by blocking key conformational changes.
- Library Optimization: The resulting library was curated for physicochemical stability, predicted immunogenicity, and developability, with a view toward advancing lead peptides into preclinical pipelines.
Our solution
We successfully developed and delivered library of novel stapled peptide antagonists specifically designed to target a pleiotropic cytokine receptor in livestock. This solution emerged from a structure-guided discovery approach that integrated advanced molecular modelling, simulation, and iterative peptide design.
Our engineered peptides demonstrated:
- High Binding Affinity and Specificity: Leveraging structure-based docking and molecular dynamics simulations, we identified peptides that competitively bound to the cytokine interaction site, effectively disrupting cytokine-receptor signaling.
- Exceptional Structural Stability: Through the incorporation of hydrocarbon staples and conformational constraints, we preserved the α-helical structure of the peptides in physiological conditions, overcoming common stability challenges seen in linear peptides.
- Tailored for Livestock Application: The final peptide library was optimized not only for biophysical and biochemical properties but also for cross-species efficacy, ensuring adaptability and translational potential across relevant livestock species.
This targeted, modular, and computationally validated peptide solution addressed both efficacy and stability challenges, providing a strong foundation for the development of next generation immunomodulators in veterinary medicine.
Key performance outcomes
Validated library of 6 novel stapled peptides, with high binding affinity and structural stability, tailored for livestock applications.
Accelerated the discovery timeline by 6–12 months: By enabling rapid in silico reconstruction of key structural features and minimizing experimental iterations.
Reduced early R&D costs by ~ 30–50%: The pre-validated library increased the functional hit rate by up to 3-fold.
Improving both candidate quality and development efficiency: By engineering conformationally stable peptides and applying cross-species modelling, the risk of downstream failure and species-specific variability was significantly reduced.
Strategic business impacts
- De-risked Development in Data-Scarce Environments: Successfully reconstructed key cytokine-receptor interactions without experimental structures, enabling targeted therapeutic design and faster decision-making.
- Improved Translational Potential: Delivered structurally robust peptides optimized for livestock physiology, ensuring efficacy and adaptability across multiple species.
- Strengthened Pipeline Efficiency: Established a scalable, structure-guided discovery framework that can be leveraged for future peptide programs targeting complex immunological pathways.
- Enabled Next-Generation Veterinary Therapeutics: Provided a solid foundation for the development of novel immunomodulators to address chronic infections and improve animal health outcomes.
- Sustained Competitive Advantage: Empowered the client with innovative solutions that reduce time-to-market while maintaining scientific rigor, reinforcing their leadership in livestock therapeutics.
Conclusion
Excelra successfully addressed the client’s challenges through a structure-guided, simulation-driven discovery approach tailored for data-scarce environments. Using homology modelling, molecular dynamics simulations, and cross-species analyses, we reconstructed key cytokine-receptor interactions and engineered conformationally stable stapled peptides, overcoming major stability and efficacy hurdles. We delivered a computationally validated library of 6 novel peptide antagonists optimized for livestock applications, demonstrating high binding affinity and cross-species relevance. This approach accelerated discovery timelines by 6–12 months, increased functional hit rates by up to threefold, reduced early R&D costs by 30–50%, and minimized species-specific risks by 20–30%. The project showcases Excelra’s capability to transform limited structural information into robust, scalable therapeutic solutions for complex veterinary immunological targets.

