Overview
A U.S.-based microbiome research company focused on developing innovative products to set new benchmarks in animal health sought to create a novel peptide-based therapeutic for feline diabetes. Feline Diabetes Mellitus (FDM) is a common endocrine disorder in cats and shares similarities with type 2 diabetes in humans, particularly in the link between obesity, insulin resistance, and disease progression. However, the precise molecular mechanisms underlying impaired insulin secretion and signaling in cats remain largely unknown.
Our client
Our client is a U.S.-based, cutting-edge biotechnology firm specializing in microbiome-driven innovations for animal health. They leverage advanced molecular design and bioinformatics to develop next-generation therapeutics. As part of a strategic initiative, the client engaged Excelra to design a novel, high-affinity triple agonist peptide by repurposing a known human therapeutic for feline use.
Client’s goals
A human peptide with agonist activity for three receptor proteins has demonstrated efficacy in both preclinical and clinical settings. The client partnered with Excelra to redesign this peptide into a novel triple agonist that targets the feline equivalents of these receptors—aiming to establish a first-in-class therapy for feline diabetes. This endeavor required precise molecular engineering to optimize specificity and therapeutic efficacy.
Our approach
We employed a comprehensive, multi-step computational modeling and bioinformatics pipeline integrating both structure- and ligand-based techniques to design and validate the engineered peptides.
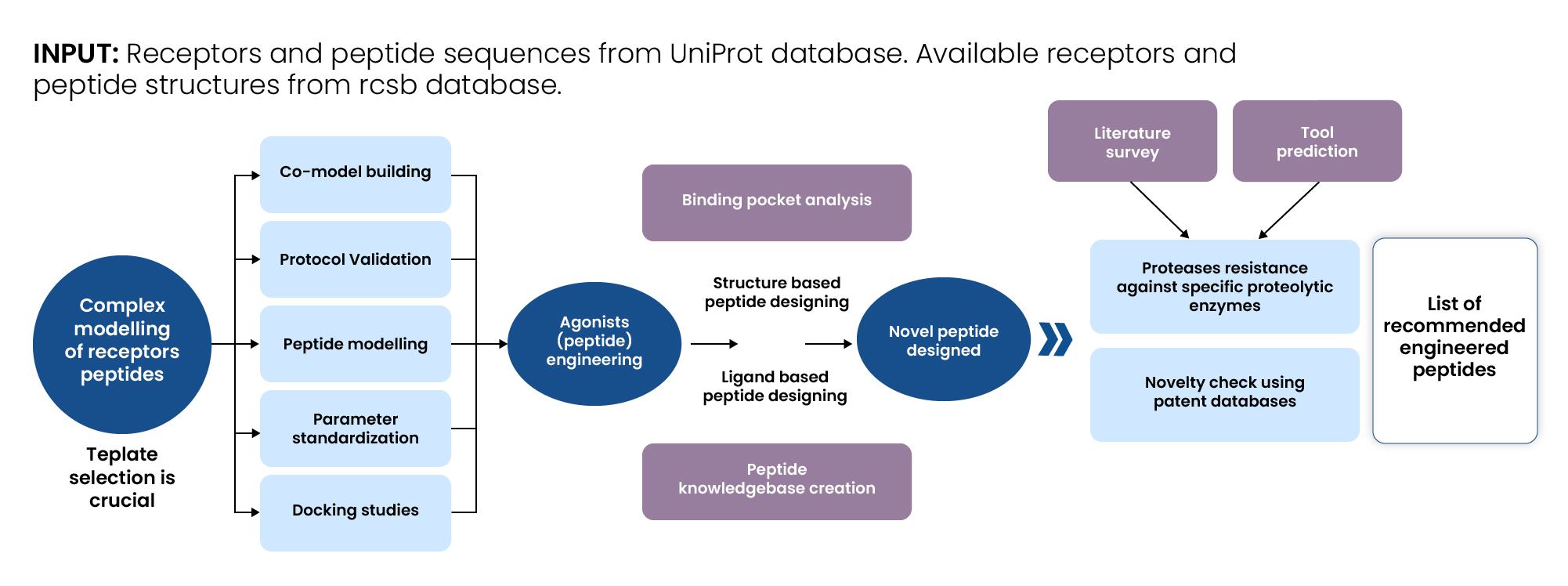
- Homology Modelling of Feline Receptors: Developed homology models of feline receptors using human crystal structures as templates. Primary, secondary, and tertiary structural analyses were conducted to ensure accuracy.
- Binding Pocket Analysis: Modelled the existing human peptide and analyzed receptor binding sites using molecular docking. Reference data was obtained from UniProt, the RCSB Protein Data Bank, and other resources.
- Peptide Agonist Knowledgebase: Compiled a database of known mono-, dual-, and tri-agonists for the target proteins, including data on structure, ADME properties, efficacy, and stability.
- Novel Peptide Design/Redesign: Used insights from binding interactions and structure-activity relationships to propose amino acid substitutions and modifications that could enhance receptor binding and peptide stability.
- Feature Analysis: Identified key data features critical to successful peptide redesign, such as hydrophobicity, charge, and receptor-binding domains.
- Peptide Design Recommendation: Synthesized data from Activities I–V to generate and recommend a set of optimized peptide candidates.
Our solution
We delivered a final panel of engineered triple agonist peptides with the following outcomes:
- Custom homology models of feline receptors facilitated structure-guided peptide design in the absence of feline crystal structures.
- Comparative structural insights enabled rational cross-species therapeutic repurposing.
- Molecular docking and interaction mapping identified key receptor-binding regions and interaction hotspots.
- Evaluation of synthetic amino acids informed modifications to improve peptide stability and receptor affinity.
A final peptide candidate was recommended based on comprehensive functional, structural, and therapeutic analyses, advancing the project towards in vitro validation. Over five high-affinity peptides with favourable docking scores were shortlisted for preclinical evaluation. R&D timelines were reduced by up to 50%, and discovery costs decreased by 30–35%, significantly accelerating lead identification and optimization.

Conclusion
Excelra’s computational approach enabled the design of over five high-affinity peptide candidates, reducing early R&D timelines by up to 50% and cutting discovery costs by 30–35% required for disease target identification, lead identification, and optimization. The solution leveraged human therapeutic protein metadata to develop animal therapeutics, significantly accelerating the drug discovery cycle by repurposing existing human drugs for veterinary use.

