Small interfering RNA (siRNA) and oligonucleotide (oligo) therapeutics are redefining precision medicine by offering unprecedented potential to silence disease-causing genes. Molecular therapeutics such as gene knockdown studies and antisense therapy are transforming the therapeutic landscape at a rapid rate. As with any promise, there is a cost—especially when managing the complex workflows associated with designing, synthesizing, screening, and analysing oligo-based therapeutics. This is where digitalization has the potential to kick in through bioinformatics solutions and scientific informatics approaches.
Excelra, as a company that enables digital revolutions in pharmaceutical R&D to deliver better scientific outcomes and accelerate drug discovery, has first-hand experience of the challenges and singular opportunities presented by siRNA and oligo therapeutics. The intent of this blog is to identify where scientific data management and digital solutions have impacted, where the key gaps remain, and map the landscape as it stands today.
The Case for Digitalization in Oligo Therapeutics
Oligo and siRNA processes are data-centric and often cross-functional between biology, chemistry and informatics. Some of the main activities are:
- Target gene silencing-based sequence designing
- Synthesis protocol and purification steps management
- Monitoring plate formats and sample movement
- In vivo and in vitro screening
- Assay result analysis and visualization
Digital platforms such as ELNs (Electronic Lab Notebooks), LIMS (Laboratory Information Management Systems), and cheminformatics software may also automate these processes by providing structured data capture, versioning, traceability, and instrument and analytics integration. These tools are central to building robust bioinformatics solutions for siRNA and oligo workflows.
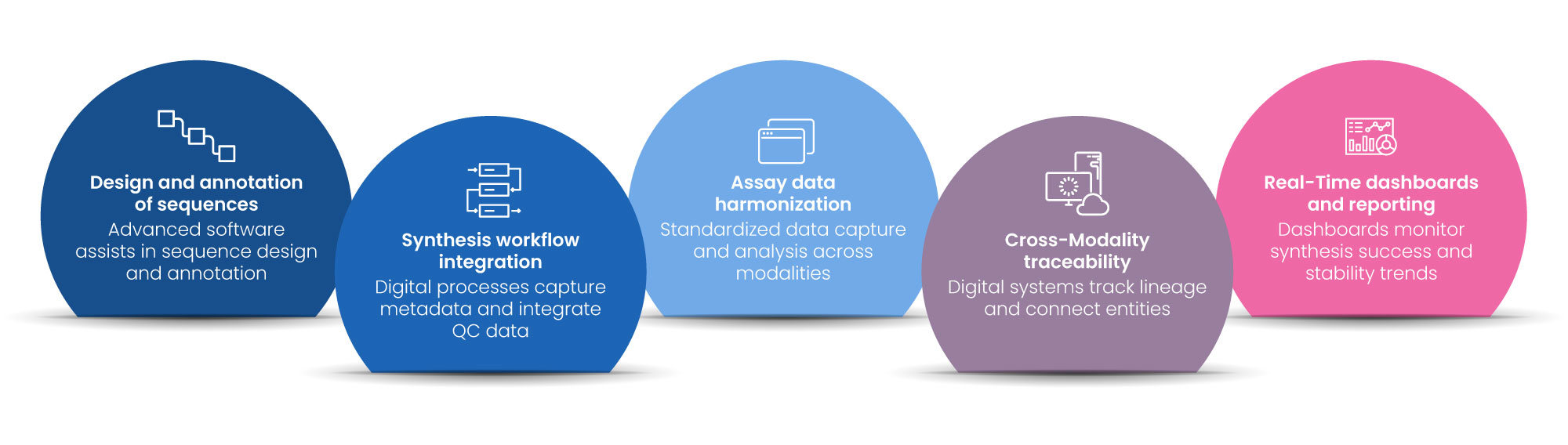
Key Opportunities for Digitalization
1. Design and Annotation of Sequences
Advanced software can assist with automated siRNA or antisense sequence design, integrate off-target prediction algorithms, and associate annotations with external databases (e.g., Ensembl, RefSeq). This enhances design accuracy and reproducibility, forming the backbone of effective scientific informatics and bioinformatics solutions in early-stage research.
2. Synthesis Workflow Integration
Oligo synthesis is usually automated with synthesizers, but digital capture of metadata—plate layout, sequence, yield, coupling efficiency—is still in development. Digital processes can facilitate batch traceability and integrate synthesis QC data with experimental records. Here, scientific data management
combined with cheminformatics platforms plays a vital role in ensuring end-to-end traceability.
3. Assay Data Harmonization
One of the most significant digitalization opportunities is the harmonization of assay data across modalities—qPCR, ELISA, HPLC, bDNA assays, and so on. ELNs’ configurable templates can standardize data capture, analysis, and visualization, reducing manual transcription and enhancing comparability. Incorporating bioinformatics solutions into this layer enables better reproducibility and collaboration across precision medicine programs.
Related reading: Structured and Analysis-Ready Data for AI/ML-Based Drug Discovery
4. Cross-Modality Traceability
In therapeutic workflows, it’s essential to track lineage from parent siRNA/oligo sequences through to their conjugates, modifications (e.g., GalNAc, PEGylation), and test results. Digital systems can connect these entities across experiments and batches using unique IDs and relational data structures, aligning with FAIR data principles for effective scientific informatics.
5. Real-Time Dashboards and Reporting
After the data is organized, the following horizon is tapping into analytics—dashboards monitoring synthesis success rates, purity profiles, biological potency, and trends in stability can enable better decision-making and accelerated candidate choice. This strengthens both scientific data management practices and long-term impact on precision medicine.
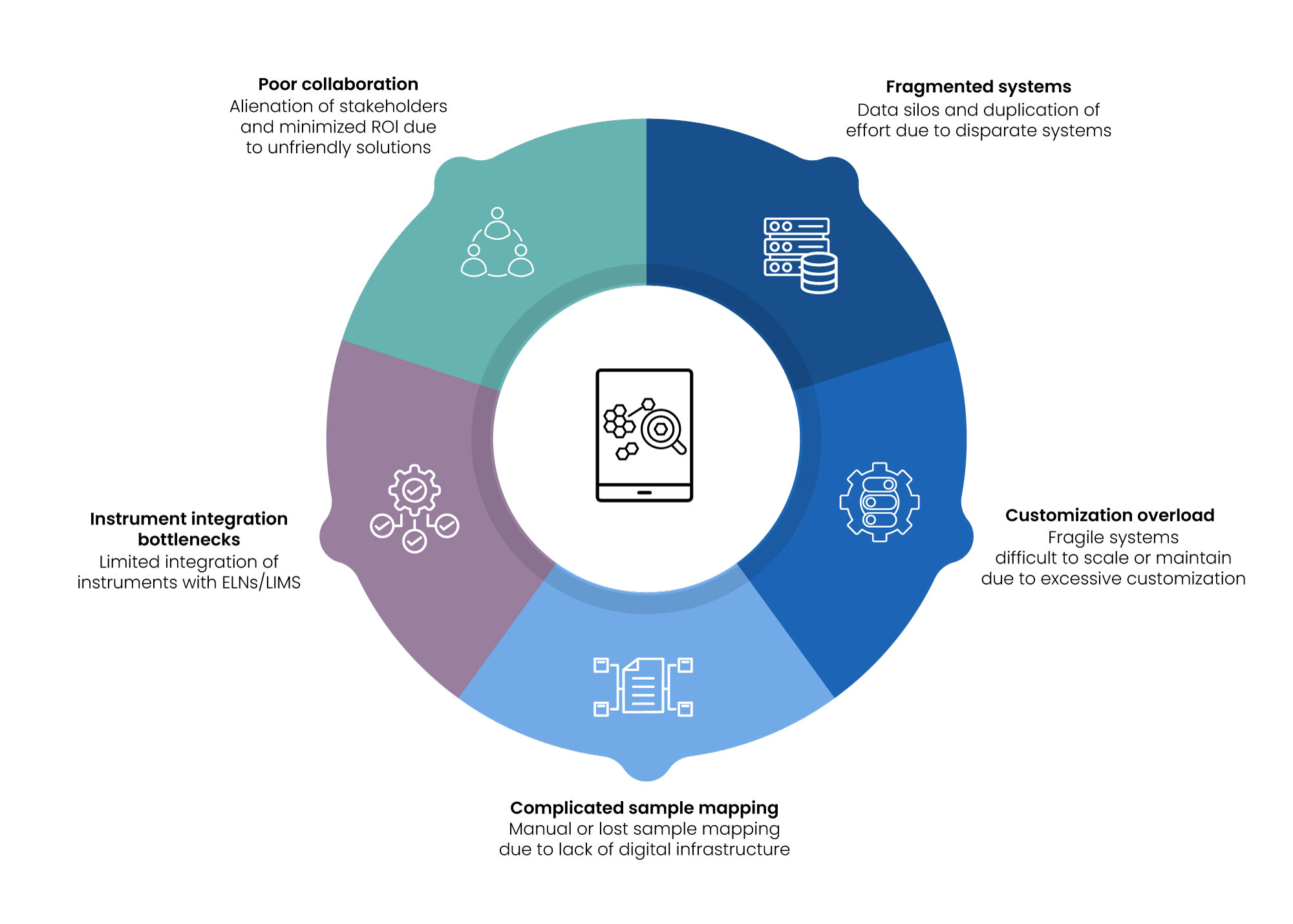
Gaps that Still Persist
1. Fragmented Systems
Most organizations still use spreadsheets and stand-alone systems for the collection of synthesis and assay data. This creates information silos, duplication of effort, and error-prone processes. A consolidated data model with integrated bioinformatics solutions is usually lacking.
2. Customization Overload
Digitalization initiatives often need to be customized to accommodate the nuances of siRNA workflows. If left unmanaged, this can result in fragile systems that are difficult to scale or maintain—hindering adoption of scientific informatics approaches.
3. Complicated Sample Mapping
Management of multiplexed or pooled siRNAs, modifications or conjugate formulations needs extensive mapping among experimental design and physical samples. Without proper digital infrastructure, this mapping is usually captured manually or lost altogether, limiting the effectiveness of scientific data management.
4. Instrument Integration Bottlenecks
Synthesizers and analytical platforms produce rich metadata, but integration with ELNs/LIMS is still limited. Parsing and linking raw data files to the correct entities and experiments remains semi-manual. Broader use of cheminformatics and bioinformatics solutions can help address these gaps.
5. Poor Collaboration Across Teams
Digital solutions that are not user-friendly or cross-functional can alienate key stakeholders—synthetic chemists, assay biologists, data analysts—resulting in partial adoption and minimized ROI. Building intuitive platforms based on scientific informatics ensures higher adoption and measurable value in precision medicine.
Where Do We Go From Here?
Building a user-friendly, extensible digital platform for complex therapeutic procedures such as siRNA and oligo research is the way forward. In order to succeed, we need to:
- Co-design workflows with scientists (end-users), not IT teams alone
- Emphasize modular, low-code digital platforms
- Artificial Intelligence can help with error identification, metadata tagging and sequence design
- From the very outset, promote FAIR data principles for sustainable scientific data management
Most probably we are just beginning to explore the possibilities of this area. With improved digital tools, intelligent integration strategies, and advanced bioinformatics solutions, we can realize the true potential of oligo therapeutics and accelerate their journey to the clinic.


